Review ArticleGastrointestinal Cancer
Liver cancer turf wars
Images
Hepatocellular carcinoma (HCC) is a common diagnosis and problem worldwide: In males it is the 5th most frequent cancer, and in women it is the 7th.1 The incidence of HCC in the United States continues to rise, and in 2011 it reached 6.2 cases per 100,000.2 From the 1970s to 2000s, overall survival increased significantly (2 vs. 8 months). As expected, the survival improvement was predominantly noted in patients with localized disease (3 vs. 18 months),2 reflecting diagnosis at earlier disease stages through screening high-risk populations with cirrhosis and the emerging broad arsenal of effective local and systemic treatment options.
Many patients with underlying cirrhosis have impaired liver function, and the degree of this dysfunction dictates prognosis as well as treatment options. The “best players” with preserved liver function and with early stage disease could benefit the most from liver transplantation, which not only treats the cancer but the underlying liver disease. However, there is a substantial wait time for transplantation and it is not unusual that many patients progress while waiting for the procedure. Another treatment option for patients with localized HCC and preserved liver function is a partial liver resection, which does not require a waiting period. Some advocate this in place of transplant but it is controversial, and is a turf war beyond the scope of this article. Unfortunately, most patients are not suitable for any surgical intervention either due to extensively disseminated intrahepatic HCC, vascular invasion, insufficient liver functional reserve, or other medical contraindications. For this population, treatment options may include localized ablative techniques such as radiofrequency ablation (RFA) and stereotactic body radiation therapy (SBRT); regional transarterial embolization techniques most commonly with chemotherapy or radiation (Yttrium-90); and systemic therapy with sorafenib, as well as combination therapy. The treatment modalities are evolving faster than level I evidence, suggesting challenges in determining the superiority of any one technique over the other. Thus, therapeutic approaches tend to vary based on institutional expertise, causing liver cancer turf wars between experts in different specialities, even in institutions with multidisciplinary panels. This review is aimed at better defining the roles for surgery, radiation oncology and interventional radiology, based on current data.
Surgery
Liver transplantation
Liver transplantation is an excellent treatment for a highly selective cohort of patients, since in the proper situation it can both cure the HCC and cirrhosis simultaneously. For decades, the Milan criteria3 (a single HCC ≤5 cm or multiple HCC 3 nodules ≤3 cm each with no macrovascular invasion or extrahepatic disease) have been used for optimal patient selection worldwide, with an overall survival rate of 75% and the recurrence-free survival of 83%.3 Several institutions are stretching this standard practice with expanded transplant criteria or by downstaging patients with encouraging results. However, these potentially expanded criteria are still in flux and need to be validated.4-10
Partial hepatectomy
Liver resection is indicated in noncirrhotic patients or patients with well-compensated cirrhosis and stage I-II disease. With limited perioperative morbidity and mortality, modern surgical techniques can achieve 5-year survival rates of at least 50%.11 In patients with very early disease (single lesion ≤2-3 cm), partial hepatectomy has yielded outcomes similar to transplantation in several retrospective series.12,13 Unfortunately, tumor recurrence rates in the remaining liver remain high (up to 80-100%14,15) due to the underlying cirrhosis, so patients often need multiple treatment strategies over a lifetime. This high recurrence rate can result in a potential turf war between transplant surgeons and surgical oncologists or hepatobiliary surgeons, which is beyond the scope of this article.
Local ablative treatment options
Radiofrequency ablation
When tumors are localized, focal treatments are preferred to minimize the risk of collateral damage in an already diseased and poorly functioning liver. RFA, performed percutaneously or intraoperatively, is a common treatment for unresectable HCC or medically inoperable patients. Efficacy is best for small tumors, less than 3-4 cm.16-19 For these patients, local recurrence rates range between 0% and 26%.20,21 Larger tumors are a bit more of a challenge, requiring several insertions to achieve complete ablation, if possible. RFA is rather convenient, typically a single outpatient treatment. On the other hand, it is an invasive procedure with placement of needle electrodes directly into liver tumors and requires anesthesia. Additionally, based on the tumor location, RFA also carries a small risk of injury to nearby structures including the lung, stomach, bowel, gall bladder and heart. Tumors near the diaphragm are difficult to visualize with ultrasound for targeting, and tumors near large vessels often cannot be fully heated, leading to incomplete treatment.
Radiotherapy
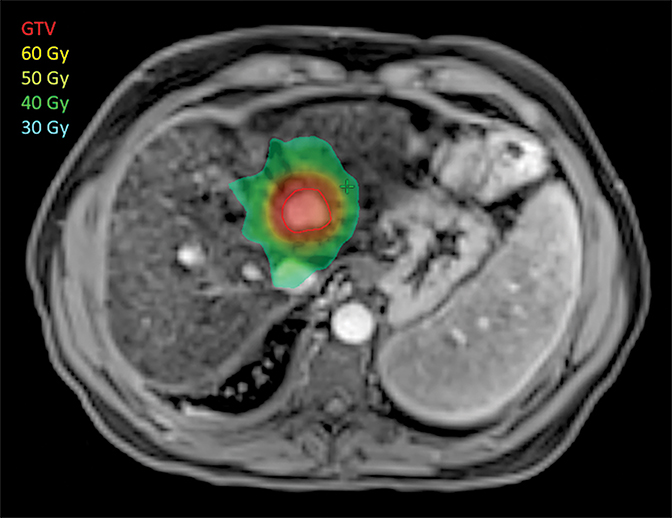
Liver SBRT (Figure 1) is a relatively new technique, which has been refined over the past decade, taking advantage of the explosion of new technologies for treatment planning, targeting and delivery. It is a non-invasive treatment that delivers high doses of precisely targeted radiation to tumors while avoiding nearby organs. Rather than extending over weeks like conventional radiotherapy, SBRT is completed in a few (1-5) treatments.
SBRT has come a long way since the first reports in the early 1990s.22 In 2008, Tse and colleagues published a phase I study of 31 patients with primary intrahepatic tumors treated with SBRT. Treatment was well-tolerated, with 17% of patients declining from Child-Pugh class A to B at 3 months, and 65% local control at 1 year.23 The phase II extension of this study to 102 patients recently demonstrated 87% 1-year local control.24 Numerous retrospective reviews have been published also demonstrating high local control rates.25-27 Toxicity has been variable, and can include liver failure and GI bleed, highlighting the importance of patient selection, careful treatment planning, dose selection (based on liver function) targeting and delivery, which should not only seek to cover the tumor with high-dose radiation, but also prioritize avoiding adjacent normal tissues. Multiple methods have emerged to assess treatment safety, from mean normal liver dose to more complicated normal tissue complication probability models. Whichever method is used, safety is stressed first, as patients with HCC typically also have cirrhosis and tenuous liver function. Dose is attenuated when necessary depending on normal liver volume. Indeed, many would advocate treating only Child-Pugh class A patients, although select B patients can be treated very carefully, preferably in a clinical trial.
In addition to treatment planning, image guidance and treatment delivery must also be meticulous. Rather than relying on external surrogates, which correlate poorly with internal tumor position, alignment is typically performed with either implanted fiducials or injected lipiodol and planar imaging, or cone-beam CT, which allows for simultaneous visualization of the tumor region and adjacent normal tissues, so potential tradeoffs between tumor coverage and normal tissue protection can be assessed. Motion management is an important component of the process. Radiated liver volumes are minimized in patients who can tolerate breath holds. For those who cannot, 4DCT can help ensure full coverage of a moving tumor.
New on the horizon is an interest in treating patients with worse hepatic function, particularly those with aggressive tumors that would otherwise progress more quickly than the patient’s liver failure. For these patients, the balance between tumor control and safety is especially difficult, and treatment is generally less aggressive to preserve safety. In a recent trial of Child-Pugh class B-C patients, 1-year local control was 55%, with 58% of patients experiencing a worsening of CP score at 1 month.28 Rather than decrease treatment intensity for all patients to maintain safety, the University of Michigan is aiming to customize treatment based on individual tolerance to therapy, using blood and imaging biomarkers to assess the liver’s response to the first 3 treatments, adjusting the last 2 treatments to maintain safety.29,30 Local control and safety have both been preserved well over 90%, even in CP B patients. Proton therapy also is a promising advance, since the low dose radiation region is dramatically reduced. Still, the potential technical limitations of proton therapy mandate that comparative clinical trials be conducted.31
SBRT for HCC is still mainly confined to academic centers, although through clinical trials such as RTOG 1112 discussed below, community centers have the opportunity to become credentialed in planning and delivery. When properly delivered, SBRT is very safe and effective. In a large single-institution review, SBRT had similar local control and less toxicity than RFA. Indeed, for larger tumors, SBRT had better results.32 Thus, at the very least, SBRT is an excellent alternative treatment when RFA is not possible or would be high-risk. A randomized trial is definitely warranted to directly compare these modalities.
Other ablative therapies
In addition to RFA and SBRT, other ablative therapies are offered in some centers. Percutaneous ethanol injection (PEI) has mostly fallen by the wayside, as multiple randomized trials have demonstrated superior tumor control with RFA.33-34 A recent meta-analysis suggests that RFA is also superior to cryoablative therapy.35 Irreversible electroporation is a new technology that has not been fully tested or compared with existing options, but could potentially be added to the growing arsenal of effective treatments in the future.
Regional ablative treatment options
Transcatheter arterial chemoembolization (TACE)
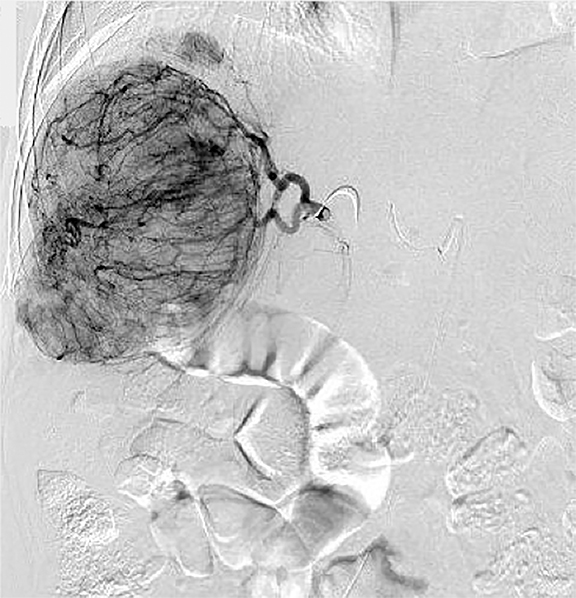
If local therapies are not available or the patient has too many tumors for safe treatment, regional therapies should be pursued (Figure 2). Response rates are generally not as high as local ablative therapies, but regional therapies can simultaneous treat numerous tumors. The main goals of TACE36,37 are: 1. Primary treatment of multinodular HCC. 2. Downstaging of large liver tumors for later transplantation or resection. 3. Palliation of pain, bleeding and arteriovenous fistula caused by the tumor. The best—but not exclusive—TACE candidates are patients with relatively preserved liver function, lesions ≤ 5cm without portal trunk thrombosis, and tumor burden occupying less than 70% of the liver. The effectiveness decreases with increasing tumor size. In the series of over 8,500 patients 1-, 3-, 5-, and 7-year survival rates following TACE were 82%, 47%, 26%, and 16%, respectively.38 Modern Drug Eluting Beads TACE (DEB-TACE) compared to conventional lipidol containing TACE39 showed higher rates of complete response (27% vs. 22%), objective response (52% vs. 44%), and disease control (63% vs. 52%), although overall survival was similar. For best results, TACE typically must be delivered repeatedly. Post-embolization syndrome consists of mild, transient nausea; fever; and abdominal pain that typically requires overnight hospitalization for observation and pain management. A transient mild decompensation in liver function is common, but acute liver failure is seen in less than 3% of procedures. Gastrointestinal and biliary events are not common. Rare serious complications include liver abscesses and vascular injury from repeated procedures.
Radioembolization (RE)
Radioembolization is a newer treatment option, aiming to combine the embolic effect of particle injection with radiation. Tumor response rates for this microsphere therapy vary between 40% and 90%, and overall disease control rates are as high as 80% in highly selective populations.40 The response is usually observed in 2-6 months. No randomized controlled trial comparing RE with other modalities has been published yet, but in large prospectively studied cohorts, intermediate stage patients treated by RE reach a median survival of 16-18 months.41-43 Side effects are similar to TACE, except for substantially less pain and potentially longer lasting fatigue, particularly in older patients.
Bridging and downstaging
Any of the above therapies can be used for bridging and downstaging, allowing successful liver transplantation or resection in selective groups. Long-term survival ranges between 49% to 92% in series describing different neoadjuvant approaches.44-47 Of note, retrospective series have demonstrated the feasibility of SBRT (35-54 Gy in 3, 50 Gy in 5) as a bridge to transplant48 to prevent progression beyond Milan Criteria while on the wait list. No intraoperative or long-term complications have been noted.
Systemic treatment
Sorafenib, an oral tyrosine kinase inhibitor, has demonstrated a small survival benefit (10.7 vs. 7.9 months) in patients with unresectable HCC with CP A liver reserve.49 The vast majority of patients were previously treated with different modalities prior to Sorafenib initiation; but unfortunately, due to lack of available local and regional therapies, many centers prescribe the drug upfront.
Combination therapies
Several rationales are behind combination therapies for HCC. First, regional and local therapies could be combined: Since regional therapies are usually not completely effective, perhaps the combination of a local therapy could improve overall response. Alternatively, local therapy to tumor thrombus in the portal vein may open the door for regional therapies. Second, systemic and local or regional therapies could be combined: Systemic therapy could be adjuvant or suppressive, or in the case of advanced disease, local therapy could be used to prevent progression-related morbidity and mortality.
Multiple randomized controlled trials have evaluated the efficacy of TACE added to RFA, compared with RFA alone. A meta-analysis involving 598 patients suggested that combination therapy had higher overall survival (OR3-year = 2.65, P < 0.001) and recurrence-free survival rate (OR5-year = 2.26, P = 0.0004) compared with RFA alone for study patients.50 Prospective studies and a meta-analysis also have reported improved survival results when radiotherapy is added to TACE.51-53 Indeed, RT to portal vein tumor thrombus is effective approximately 35% of the time,54 which could make patients eligible for regional therapies. The opposite study of whether TACE improves the outcome after RT has not yet been performed.
Any local treatment can cause upregulation of circulating vascular endothelial growth factor(VEGF)—thus, the rationale behind combining ablative therapy with antiangigenetic therapy. A meta-analysis with a total of 1,254 patients favored the combination of TACE with sorafenib in terms of significantly improved overall survival (OS) (hazard ratio [HR] = 0.65, P = 0.007), time to progression (TTP) (HR = 0.68, P = 0.003), and overall response rate (ORR) (HR = 1.06, P = 0.021), but did not affect progression-free survival (PFS).55 The combination therapy was generally well-tolerated but, as expected, had more side effects related to TKI compared with observation alone—mostly fatigue, diarrhea and skin changes. The addition of sorafenib to RFA and RE for intermediate and advanced stage patients is being explored in randomized controlled trials.
Another important clinical question is whether adding local treatment to systemic treatment in intermediate and advanced HCC could improve overall outcome. This hypothesis is tested in RTOG 1112, an ongoing international phase III study of sorafenib vs. SBRT followed by sorafenib. Another ongoing phase III trial STOP-HCC evaluates the efficacy of RE added to sorafenib.
Conclusion
Whenever suitable, surgical options should be considered as a gold standard. Otherwise, based on the data above, clinicians can propose several treatment options in almost every clinical setting. Unfortunately, Level I evidence to guide decisions is lacking in most situations. Generally, we prefer discussion over argument in the absence of data, but are there certain patients we should strongly advocate for? The most important question that we, as radiation oncologists, should ask ourselves is: Which patients would benefit the most from SBRT—i.e., who should we fight for on the tumor board battlefield? This question answers itself if we consider the main advantages of SBRT: It is highly effective, noninvasive and relatively safe, even in situations and geometries that would be relatively high risk for other treatments. We propose the following scenarios where SBRT could be considered favorably:
If local ablative treatments such as RFA are under consideration, SBRT is preferred if the tumor is >3 cm (likely incomplete RFA) at the liver dome (poor visualization by ultrasound makes RFA difficult), in a close proximity to major vessels (poor heating due to the heat sink leads to incomplete RFA), gallbladder, or gastrointestinal tract (potential for perforation).
When other modalities could pose danger if the patient has certain medical conditions (such as thrombocytopenia or is at high risk for anesthesia), SBRT has a very favorable risk-benefit profile.
SBRT can be considered following TACE or RE with mixed response (e.g., 1-3 growing lesions). SBRT of single lesions following RE failure can be considered if other local ablative treatment modalities are not appropriate.
If portal vein thrombosis is making regional therapy high-risk, SBRT should be strongly considered. Depending on the size and number of tumors, treatment can be directed at all disease. Another option would be SBRT aiming to open the portal vein to make the patient a candidate for additional treatment modalities.
Despite these scenarios, we should keep in mind that SBRT requires appropriate treatment planning, delivery and image-guidance equipment, as well as the expertise of radiation oncologists, physicists, dosimetrists and therapists. RTOG/NRG guidelines and protocols, training workshops, and fellowships aim to help centers develop SBRT programs and bring this treatment option to more patients worldwide.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
- Njei B, Rotman Y, Ditah I, et al. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61(1):191-199.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-699.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394-1403.
- Onaca N, Davis GL, Goldstein RM, et al. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2007;13(3): 391-399.
- Takada Y, Ito T, Ueda M, et al. Living donor liver transplantation for patients with HCC exceeding the Milan criteria: a proposal of expanded criteria. Dig Dis. 2007;25(4):299-302.
- Soejima Y, Taketomi A, Yoshizumi T, et al. Extended indication for living donor liver transplantation in patients with hepatocellular carcinoma. Transplantation. 2007;83(7):893-899.
- Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14(7):935-945.
- Silva M, Moya A, Berenguer M, et al. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl. 2008;14(10):1449-1460.
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35-43.
- Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25(2):181-200.
- Adam R, Bhangui P, Vibert E, et al. Resection or transplantation for early hepatocellular carcinoma in a cirrhotic liver: does size define the best oncological strategy? Ann Surg. 2012;256(6):883-891.
- Sapisochin G, Castells L, Dopazo C, et al. Single HCC in cirrhotic patients: liver resection or liver transplantation? Long-term outcome according to an intention-to-treat basis. Ann Surg Oncol. 2013;20(4):1194-1202.
- Fuster J, García-Valdecasas JC, Grande L, et al. Hepatocellular carcinoma and cirrhosis. Results of surgical treatment in a European series. Ann Surg. 1996;223(3):297-302.
- Sasaki A, Iwashita Y, Shibata K, et al. Improved long-term survival after liver resection for hepatocellular carcinoma in the modern era: retrospective study from HCV-endemic areas. World J Surg. 2006;30(8):1567-1578.
- Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201.
- Sala M, Llovet JM, Vilana R, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352.
- Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82.
- Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10(2 Suppl 1):S115-20.
- Ikeda K, Seki T, Umehara H, et al. Clinicopathologicstudy of small hepatocellular carcinoma with microscopicsatellite nodules to determine the extent of tumor ablationby local therapy. Int J Oncol. 2007;31:485-491.
- Lam VW, Ng KK, Chok KS, et al. Risk factors and prognostic factorsof local recurrence after radiofrequency ablation of hepatocellularcarcinoma. J Am Coll Surg. 2008;207:20-29.
- Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34(6):861-870.
- Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26(4):657-664.
- Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31(13):1631-1639.
- Liu E, Stenmark MH, Schipper MJ, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors. Translational Oncology. 2013;6; 442-446.
- Choi BO, Choi IB, Jang HS, et al. Stereotactic body radiation therapy with or without transarterial chemoembolization for patients with primary hepatocellular carcinoma: Preliminary analysis. BMC Cancer. 2008; 8:351.
- Van der Pool AE, Méndez Romero A, Wunderink W, et al. Stereotactic body radiation therapy for colorectal liver metastases. Br J Surg. 2010; 97:377-382.
- Culleton S, Jiang H, Haddad CR, et al. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014;111(3):412-417.
- Normolle D, Pan C, Ben-Josef E, et al. Adaptive trial of personalized radiotherapy for intrahepatic cancer. Per Med. 2010;7(2):197-204.
- Feng M, Schipper MJ, Balter JM, et al. A phase 2 trial of individualized adaptive stereotactic body radiation therapy (SBRT) for patients at high risk for liver damage. Int J Radiat Oncol Biol Phys. 2013:87(2),S27.
- Lawrence TS, Feng M. Protons for prostate cancer: the dream versus the reality. J Natl Cancer Inst. 2013;105(1):7-8.
- Liu E SM, Schipper MJ, Caoili EM, Ben-Josef E, Lawrence TS, Feng M. SBRT as an Alternative to RFA for the Treatment of Primary and Metastatic Liver Tumors. Journal of Clin Oncol 2012; 30:158.
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228(1):235-240.
- Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol. 2008;43(6):727-735.
- Huang YZ, Zhou SC, Zhou H, et al. Radiofrequency ablation versus cryosurgery ablation for hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2013;60(125):1131-1135.
- Vogl TJ, Naguib NN, Nour-Eldin NE, et al. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol. 2009;72(3):505-516.
- EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. J Hepatol. 2012;56(4):908-943.
- Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8,510 patients. Gastroenterology. 2006;131(2): 461-469.
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224(1):47-54.
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(1):52-64.
- Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54(3):868-878.
- Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57(5):1826-1837.
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;2140(2):497-507.e2.
- Lei JY, Yan LN, Wang WT. Transplantation vs resection for hepatocellular carcinoma with compensated liver function after downstaging therapy. World J Gastroenterol. 2013; 19:4400-4408.
- Fan J, Tang ZY, Yu YQ, et al. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg. 1998;15:674-678.
- Tang ZY, Zhou XD, Ma ZC, et al. Downstaging followed by resection plays a role in improving prognosis of unresectable hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2004;3:495-498.
- Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48 819-827.
- Katz, AW, Chawla, S, Qu, Z et al. Stereotactic hypofractionated radiation therapy as a bridge to transplantation for hepatocellular carcinoma: Clinical outcome and pathologic correlation. Int J Radiat Oncol Biol Phys. 2012;83: 895-900.
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-90.
- Ni JY, Liu SS, Xu LF, et al. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;28;19(24):3872-3882.
- Shim SJ, Seong J, Han KH, et al. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005; 25:1189-1196.
- Oh D, Lim do H, Park HC et al. Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: a prospective evaluation of efficacy and toxicity. Am J Clin Oncol. 2010;33: 370-375.
- Meng MB, Cui YL, Lu Y, et al. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2009;92(2): 184-194.
- Xi M, Zhang L, Zhao L, et al. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One. 2013;8(5):e63864.
- Zhang L, Hu P, Chen X, et al. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9(6):e100305.
Citation
Sapir E, AlAlfy E, Novelli P, Feng M. Liver cancer turf wars. Appl Rad Oncol. 2015;(1):8-13.
March 11, 2015
