Organ preservation in patients with locally advanced larynx cancer: Important principles and treatment considerations
Images


CASE SUMMARY
A 63-year-old male with a 20 pack-year smoking history and a hard liquor intake of 12-16 drinks per week presented with a 5-month history of hoarseness and a sore throat. He also had a 1-month history of progressive stridor on exertion. Flexible laryngoscopy revealed a bulky, submucosally infiltrative mass with the epicenter at the right false cord with involvement of the right true cord, the right arytenoid and a fixed larynx. The superior aryepiglottic fold on the right appeared to be spared, as did the epiglottis, but the airway was significantly narrowed.
The patient underwent local and systemic imaging studies, as well as a direct laryngoscopy with biopsy and tracheostomy. Intraoperative findings included significant narrowing of the airway from extensive subglottic extension of the tumor, obliteration of the right false and true cords, and extension of the tumor to the anterior commissure. Biopsy of the right hemilarynx revealed an invasive moderately differentiated squamous cell carcinoma.
IMAGING FINDINGS
Computed tomography (CT) of the neck revealed fullness in the right true vocal fold extending across the anterior commissure, with obliteration of periglottic fat plane, and asymmetric sclerosis and lytic change in the thyroid lamina (Figure 1). There was asymmetric sclerosis of the right arytenoid cartilage and mild asymmetric fullness of the strap muscles overlying the larynx on the right concerning for extralaryngeal spread. There was no obvious radiographic evidence for gross subglottic extension, and the subglottic airway was patent. There was no bulky adenopathy in the neck.
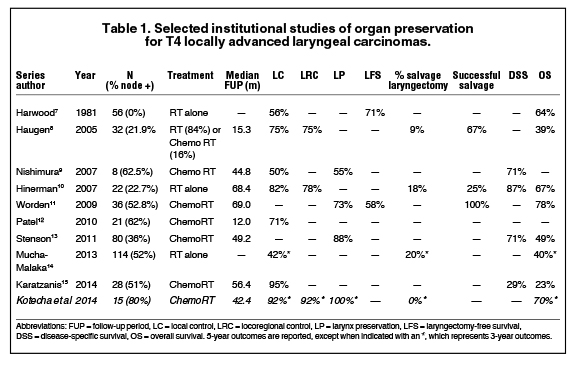
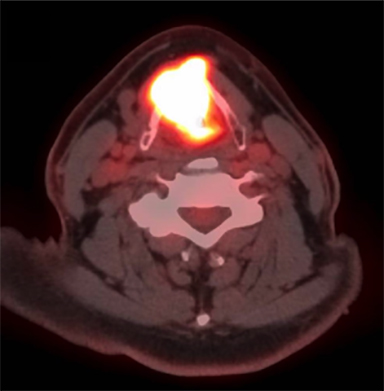
A positron emission tomography/CT (PET/CT) scan revealed a 3.5 x 3.0-cm soft tissue thickening of the right vocal cord with moderately increased fluorodeoxyglucose (FDG) uptake (Max SUV 16) consistent with neoplasm (Figure 2A). The tumor crossed midline and extended across the anterior commissure, obliterating the periglottic fat plane. It encased and eroded the right thyroid lamina. There was a 1.7 x 0.8-cm mildly FDG avid lymph node in the right level IIa (Max SUV 3.7) (Figure 2B).
DIAGNOSIS
Stage IVA T4aN1M0 locally advanced squamous cell carcinoma of the larynx.
DISCUSSION
Prior to the publication of the Veterans Affairs (VA) Laryngeal Cancer Study, the standard treatment for patients with locally advanced laryngeal cancer consisted of a total laryngectomy with postoperative radiation therapy recommended for patients with certain high-risk pathological features. The results of the VA study demonstrated that a strategy of induction chemotherapy followed by definitive radiation therapy was an effective approach for larynx preservation without compromising a patient’s overall survival compared to total laryngectomy. Despite these promising general results, 56% of patients with T4 cancers required salvage laryngectomy, compared to 26% of patients with smaller primary tumors, p=0.001.1
Consequently, patients with high-volume T4 primaries (invasion > 1 cm into the base of tongue or penetration through cartilage) were excluded in the subsequent RTOG 91-11 trial evaluating the benefit of concurrent chemoradiotherapy compared to induction chemotherapy followed by definitive radiotherapy or definitive radiation therapy alone.2 Since the publication of the VA Larynx Study and RTOG 91-11, the inclusion of this cohort of patients into randomized studies investigating voice-preserving treatment alternatives has been limited.
In addition to the requirement of a permanent tracheostomy and the morbidity of the surgical procedure, total laryngectomy is also associated with a significant detriment to a patient’s vocal communication, reducing quality of life.3 Moreover, in a survey of volunteers given the treatment option of either total laryngectomy or a laryngeal preservation protocol using chemotherapy and radiotherapy, only 24.6% of patients rated survival their main consideration (versus quality of life) if faced with advanced-stage laryngeal cancer.4 This philosophy has led to a reduction in laryngectomies in the United States by 48% over the past 10 years.5
For patients with T4 larynx cancer who decline surgery, the National Comprehensive Cancer Network guidelines recommend concurrent chemoradiotherapy, induction chemotherapy followed by response assessment, or enrollment in a clinical trial.6 Multiple institutional experiences have demonstrated modest outcomes in patients with T4 primary cancers treated with larynx-preserving approaches (Table 1).7-15 Even in these series, however, patients with thyroid cartilage invasion are underrepresented. A review of 25 patients with thyroid or cricoid cartilage invasion treated with chemoradiotherapy demonstrated that patients with cartilage invasion involving both cortices had an inferior local control compared to patients with no or minor cartilage invasion (2-year rate: 55% versus 81%, p<0.05), but no significant reduction in survival with a functional larynx or overall survival.16
When using CT imaging to determine the optimal patient management approach for patients with advanced larynx cancer, it is also important to understand the limitations of imaging accuracy in detecting thyroid cartilage penetration or extralaryngeal spread. For example, in one series of 107 laryngectomy specimens, CT imaging identified 59% and 49% of cases of pathologically documented thyroid cartilage penetration and extralaryngeal spread, respectively, with corresponding positive predictive values of 74% and 81%.17
At our institution, only select patients who are healthy enough to tolerate combined modality treatment, compliant enough for close follow-up, and motivated enough for larynx preservation are treated with concurrent chemoradiotherapy. Upon review of an institutional review board (IRB)-approved tumor registry, 15 patients with T4 disease were treated at the Cleveland Clinic from 1993-2011 with combined chemoradiotherapy. For this cohort, the local control, locoregional control, and larynx preservation rates have been 92%, 92% and 100%, respectively at 3 years (Table 1). Note, however, that one patient with a local failure was recommended to undergo laryngectomy and declined, putting the recommended larynx preservation rate at 92% as well (95% CI 77.8%-100%). While it remains uncommon for our institution to treat these patients with a non-surgical approach, these results demonstrate favorable outcomes in a carefully selected subset of patients.
Evaluation of the response to induction chemotherapy is one proposed method to better select patients with locally advanced larynx cancer for organ preservation. For example, Urba and colleagues reported a favorable larynx preservation rate of 70%, and a 3-year overall survival rate of 85%, in a population of patients selected for larynx preservation based on >50% response to induction cisplatin and fluorouracil.18 This, however, may not be the optimal method to appropriately select patients. In the Radiation Therapy Oncology Group (RTOG) 91-11 trial, of the 11 patients who had less than a partial response to induction chemotherapy but continued with additional chemotherapy or radiation therapy, all 11 had a complete response, and only one patient eventually required a laryngectomy.19 Therefore, it is important to note that the response to chemotherapy alone does not always correlate with a patient’s response to concurrent chemoradiotherapy.
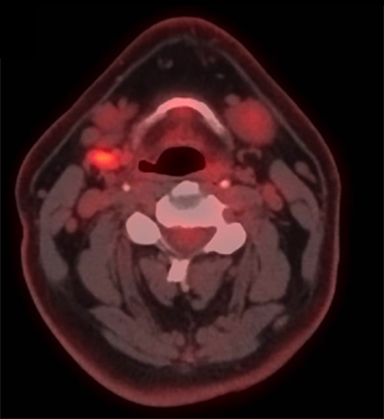
When embarking on a larynx-preservation approach in patients with locally advanced primary cancers, it is important to evaluate the need for a tracheostomy before therapy (and its coverage during treatment planning), design nodal volumes to include the at-risk nodal drainage pathways during target delineation, make use of an adaptive re-plan to reduce the treatment volume as the tumor volume shrinks, and follow the patient closely after therapy completion. One should also consider pre-treatment placement of a tracheostomy in patients with bulky tumors causing a narrowed airway that could become occluded by radiation-induced edema. For routine intensity-modulated radiation therapy (IMRT) treatment planning, the bilateral cervical nodal levels II-IV are included in all patients with supraglottic primaries or stage III/IV glottic primaries (Figure 3).20
For patients with more advanced local disease, especially with anterior extension of disease through the cartilage or subglottic extension, the level VI nodes should also be included (Figure 3I). As defined in the 2013 update of the consensus guidelines, adequate coverage of the level VI nodes includes the anterior jugular, pre-laryngeal, pre-tracheal and para-tracheal (recurrent laryngeal nerve) nodes, as well as the deep previsceral space.21 Thus, the level VI contour volume should extend from the caudal edge of the hyoid bone to the cranial edge of the sternal manubrium, limited anteriorly by the platysma and posteriorly by the anterior surface of the infrahyoid muscles. Adaptive radiotherapy has been proposed as a method of decreasing late toxicity and possibly preventing a geographical miss in patients who experience significant tumor shrinkage or weight loss during therapy. While the dosimetric benefit of adaptive re-planning is clear, whether this dosimetric benefit correlates to improved control and reduced toxicity remains to be demonstrated.22-23
Finally, in bulky T4 patients who decline laryngectomy, the importance of close follow-up with laryngoscopy cannot be overstated. The randomized data showed no survival benefit to laryngectomy, but this depends on effective salvage, which may be compromised if the patient is lost to follow-up.
To recapitulate, the patient in this report was diagnosed with a Stage IVA (T4 secondary to thyroid cartilage penetration) locally advanced laryngeal cancer. He declined surgery and was enrolled onto the RTOG 35-01 randomized trial (a phase II randomized study investigating the use of lapatinib, a tyrosine kinase inhibitor with dual action against Her-2/neu and EGFR pathways, in conjunction with standard chemoradiotherapy) and underwent concurrent chemoradiotherapy to a total dose of 70 Gy in 35 fractions, accelerated over 6 weeks, using 6 MV photons via 9-field IMRT (Figure 4). He received 44 Gy in 22 fractions with an initial IMRT plan, and the remainder 26 Gy was delivered using an adaptive re-plan due to significant tumor response as observed on flexible laryngoscopy during his OTR visit. He received two cycles of bolus cisplatin (100 mg/m2) and was also randomized to receive Lapatinib. He tolerated the treatment well with (CTCAE criteria v4.0) grade 1 dysphagia, mucositis, xerostomia, and taste changes. He experienced grade 2 fatigue, nausea, vomiting, and weight loss. He had a grade 3 voice change (whispered speech) upon therapy completion.
CONCLUSION
A total laryngectomy followed by adjuvant radiation therapy remains the conventional treatment for patients with locally advanced laryngeal cancer, especially in those with extension of disease through the thyroid cartilage. For select motivated patients who desire organ preservation, this approach can lead to modest local control without sacrificing the chance of long-term survival. Important principles in selecting these patients for a non-surgical approach include assessment for the need for upfront tracheostomy, ability to tolerate concurrent chemotherapy, and reliability for close follow-up. To optimize radiotherapy management, accurate target volume delineation should include coverage of the gross tumor volume and adequate coverage of the at-risk lymph node levels.
REFERENCES
- Group, D.O.V.A.L.C.S. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685-1690.
- Forastiere AA, Zhang Q, Randal S, Weber RS, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013; 31(7):845-852.
- Harwood AR and Rawlinson E. The quality of life of patients following treatment for laryngeal cancer. Int J Radiat Oncol Biol Phys. 1983; 9(3):335-338.
- Laccourreye O, Malinvaud D, Holsinger FC, Consoli S, Menard M, Bonfils P. Trade-off between survival and laryngeal preservation in advanced laryngeal cancer: the otorhinolaryngology patient’s perspective. Annals of Otol Rhinol Laryngol. 2012;121(9):570.
- Maddox PT, Davies L.Trends in totallaryngectomy in the era of organ preservation: a population-based study. Otolaryngol Head Neck Surg. 2012;147:85-90.
- NCCN Guidelines Version 2.2014. “Head and Neck Cancers” Available from: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- Harwood AR, Beale FA, Cummings BJ, Keane TJ, Payne D, Rider WD. T4nomo glottic cancer: an analysis of dose-time volume factors. Int J Radiat Oncol Biol Phys. 1981;7(11):1507-1512.
- Haugen H, Johansson KA, Ejnell H, et al. Accelerated radiotherapy for advanced laryngeal cancer. Acta Oncologica. 2005;44(5):481-489.
- Nishimura G, Tsukuda M, Horiuchi C, et al. Concurrent chemoradiotherapy for T4 patients with hypopharyngeal and laryngeal squamous cell carcinomas. Auris Nasus Larynx. 2007;34(4):499-504.
- Hinerman RW, Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Villaret DB. T3 and T4 true vocal cord squamous carcinomas treated with external beam irradiation: a single institution’s 35-year experience. Am J Clin Oncol. 2007;30(2):181-185.
- Worden FP, Moyer J, Lee JS, et al. Chemoselection as a strategy for organ preservation in patients with T4 laryngeal squamous cell carcinoma with cartilage invasion. Laryngoscope. 2009;119(8):1510-1517.
- Patel UA, Howell LK. Local response to chemoradiation in T4 larynx cancer with cartilage invasion. Laryngoscope. 2011;121(1):106-110.
- Stenson KM, Maccracken E, Kunnavakkam R, et al. Chemoradiation for patients with large-volume laryngeal cancers. Head Neck. 2012;34(8):1162-1167.
- Mucha-Małecka A, Składowski K. High-dose radio- therapy alone for patients with T4-stage laryngeal cancer. Strahlenther Onkol. 2013;189(8):632-638.
- Karatzanis AD, Psychogios G, Waldfahrer F. et al. Management of locally advanced laryngeal cancer. Otolaryngol Head Neck Surg. 2014; 43(1):4.
- Wagner MM, Curé JK, Caudell JJ, et al. Prognostic significance of thyroid or cricoid cartilage invasion in laryngeal or hypopharyngeal cancer treated with organ preserving strategies. Radiat Oncol. 2012;7(1):219.
- Beitler JJ, Muller S, Grist WJ, et al. Prognostic accuracy of computed tomography findings for patients with laryngeal cancer undergoing laryngectomy. J Clin Oncol. 2010;28(14):2318-2322.
- Urba S, Wolf G, Eisbruch A, et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol. 2006;24(4):593-598.
- Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003. 349(22):2091-8.
- Eisbruch A, Foote RL, O’Sullivan B, et al. Intensity-modulated radiation therapy for head and neck cancer: emphasis on the selection and delineation of the targets. Semin Radiat Oncol. 2002.Jul;2(3):238-49.
- Grégoire V, Ang K, Budach W, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014.Jan;110(1):172-81. doi: 10.1016/j.radonc. 2013.10.010. Epub 2013 Oct 31.
- Ahn PH, Chen CC, Ahn AI, et al. Adaptive planning in intensity-modulated radiation therapy for head and neck cancers: single-institution experience and clinical implications. Int J Radiat Oncol Biol Phys. 2011. 80(3):677-85.
- Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012. 83(3):986-93.
