Profound radiation pneumonitis preceding pathologic complete response in a patient with locally advanced nonsmall cell lung canc
Images
CASE SUMMARY
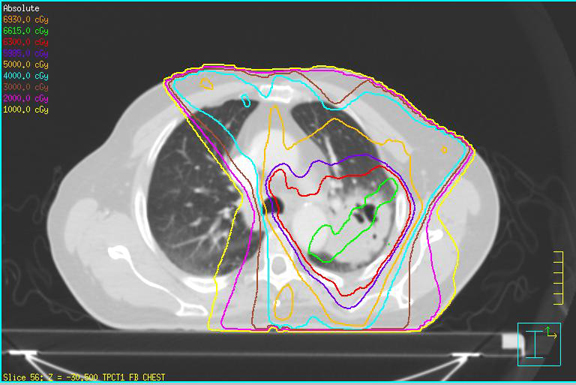
A 79-year-old man with a remote history of smoking presented with blood-tinged sputum. After imaging/workup, he was staged with T2bN2M0 (stage IIIA) squamous-cell carcinoma of the lung and was scheduled for chemoradiotherapy (CRT) and subsequent restaging/evaluation for surgical resection. He received 6,300 cGy of external beam radiation therapy (EBRT) in 35 fractions to his left lung mass, left hilar adenopathy, and paratracheal lymphadenopathy using 6-field intensity-modulated radiation therapy (IMRT) (Table 1) with weekly carboplatin AUC 5 on days 1 and 22 and weekly docetaxel 20mg/m2. He developed mild esophagitis and RTOG grade II dermatitis as well as dose-limiting thrombocytopenia, which were managed conservatively. Rapid radiographic onset of radiation pneumonitis was noted at 5 weeks after CRT (although clinical symptoms remained mild), and surgery was postponed. After 30 days of oral prednisone (20 mg/day), the patient showed marked improvement and proceeded to surgery. Surgery was well tolerated, and analyses indicated a complete pathologic response to CRT.
IMAGING FINDINGS
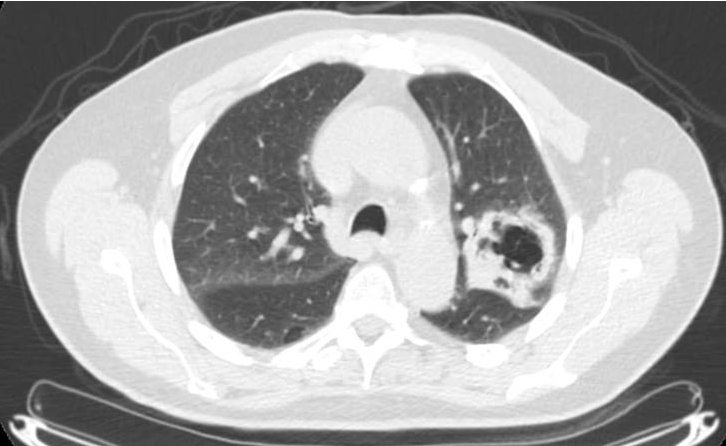
At presentation: At initial presentation, a chest radiography showed a 6.5-cm left upper lobe cavitary lesion. Computed tomography (CT)-guided biopsy of the mass demonstrated poorly differentiated squamous cell carcinoma with necrosis. Positron emission tomography/CT (PET/CT) showed a maximum standardized uptake value (SUVmax) of 14.4 in the primary and 17.3 in a left hilar node (Figure 1). Quantitative ventilation/perfusion scanning demonstrated a mismatch corresponding to the known tumor. Mediastinoscopy demonstrated poorly differentiated squamous-cell carcinoma in the 4L lymph node. The 4R, 7, and left mainstem bronchus biopsies were negative for metastatic disease. Other initial metastatic workup included magnetic resonance imaging (MRI) of the brain.
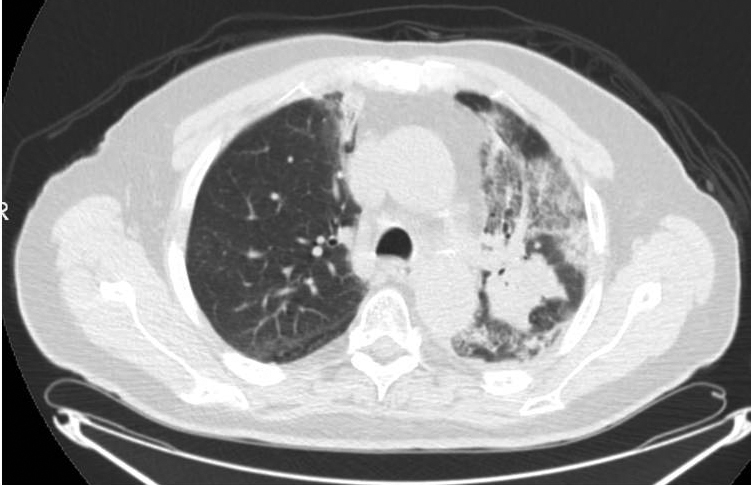
In evaluation for surgery: At 1 month after CRT, the patient noted a mild increase in shortness of breath with exertion (although able to perform 30 minutes of cardiovascular exercise 3-4 times/week) and a productive intermittent cough. Pulmonary function testing showed a forced expiratory volume of 2.82 (103% of predicted), total lung capacity of 6.01 (85% of predicted), residual volume of 2.23 (81% of predicted), and CO lung diffusion capacity (DLCO) of 14.1 (60% of predicted). Restaging PET/CT at 5 weeks after treatment showed diffuse consolidation throughout the left-upper lobe associated with intense tracer uptake (SUVmax range, 6.8-8.5) consistent with radiation pneumonitis (Figure 2).
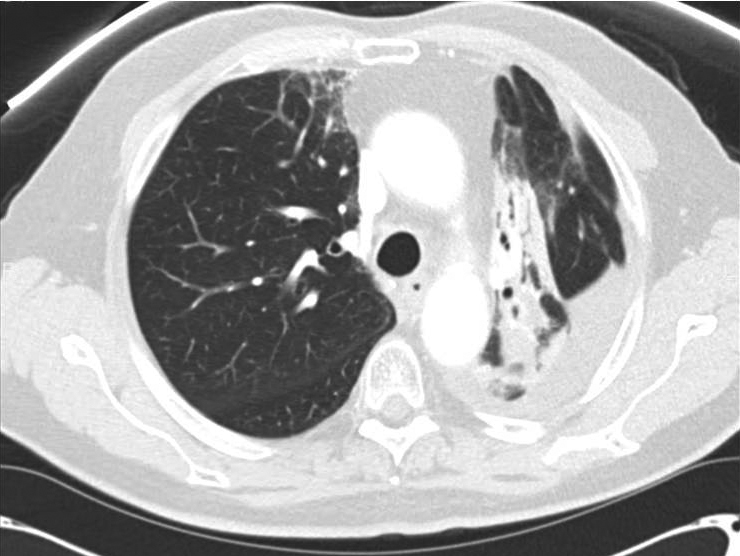
Perioperative: After 30 days of oral prednisone, CT (Figure 3) showed decreases in reactive lung changes. The patient underwent a left thoracoscopy with pleural biopsies, followed by a left thoracotomy and left upper lobectomy. On operative report, an atelectatic left lung was recognized with the parenchyma of the posterior aspect of the upper lobe and the superior segment of the lower lobe appearing dark and consolidated, consistent with areas of treated disease and pneumonitis. By comparison, the anterior aspect of the upper lobe and rest of the lower lobe appeared normal. The patient subsequently underwent a staged thoracotomy and left upper lobectomy where additional reactive adhesions of the lung to the mediastinal pleura were recognized. Fibrosis was particularly intense surrounding the aortopulmonary window and the posterior aspect of the upper lobe where the gross tumor volume was located. Changes to the lung tissue extended across the posterior aspect of the fissure to the superior segment, where the radiographically identified consolidation had extended. Thoracic lymphadenectomies were then performed at levels 5 and 7 to 12. Pathology from the resected left-upper lobe showed fibrosis, coagulative necrosis, and reactive epithelial cells with no evidence of malignancy. None of these lymph node areas showed evidence of malignancy, indicating a complete pathologic response to neoadjuvant CRT.
Follow-up: At 1 month after surgery, the patient was asymptomatic with oxygen saturation of 96% of room air.
DISCUSSION
This case of radiation pneumonitis presented with minimal symptomatic change, no identifiable predictors of rapid pneumonitic response, and lung dose–volume histogram (DVH) criteria within institutionally acceptable limits.
Radiation pneumonitis is observed clinically in 10% to 20% of patients receiving concurrent CRT for locally advanced nonsmall cell lung cancer, with a much higher percentage of patients exhibiting asymptomatic radiographic changes.1-3 Type II pneumocytes demonstrate early response to lung irradiation and endothelial damage leading to vascular congestion and capillary swelling 1 to 6 weeks after irradiation, which can clinically manifest as a reduction in DLCO on pulmonary testing, as observed in our patient.4 The acute phase of radiation-induced lung injury is transient and typically occurs within 4 to 12 weeks of completing therapy. Fibrosis occurs 6 to 12 months after completing RT and is caused by infiltration of fibroblasts into alveolar walls, which progresses to vascular sclerosis.5
Risk factors for developing clinically significant radiation pneumonitis include ECOG performance status > 1, female sex, age > 60 years, and concomitant chemotherapy.5-8 Docetaxel administration is associated with radiation pneumonitis, and weekly schedules have been associated with higher rates of pneumonitis than have administrations every 3 weeks.9
Nonsmokers also have less baseline damage to their lungs prior to RT and show higher rates of pneumonitis than smokers.10 Significant dosimetric parameters, such as V20 ≤ 25, V25 ≤ 20, V35 ≤ 15 and V50 ≤ 10, have shown to result in 2% rates of ≥ grade 3 pneumonitis and were achieved in this patient10-12(Table 1). Other than concomitant chemotherapy and advanced age, the patient had few risk factors that would have predicted the severity of his pneumonitic reaction.
Figure 2 demonstrates the isodose distribution in our plan and corresponding development of radiographic pneumonitis. Radiographic evidence of pneumonitis may appear at a marked geographic distance from the planning target volume and may be confused with infection or lymphangitic spread of neoplasm. When interpreting radiographic changes post-RT for purposes of restaging, it is vital for the radiation oncologist to work with the radiologist to describe the treatment plan and beam arrangements that may account for unusual areas of posttreatment change within the lung.5 The contralateral linear consolidation pattern observed in our patient corresponds well to beam arrangements visualized during treatment planning, supporting suspicion for radiation-induced pneumonitis.
Corticosteroids are often used to improve symptomatic pneumonitis. To our knowledge, this is the first reported case in which corticosteroids were used with the primary goal of improving the patient’s pulmonary function to increase eligibility for surgery.4,13 The patient had suffered a dramatic decline in DLCO, which made him less than ideal as a surgical candidate.14,15 A 1-month trial of oral corticosteroids resulted in radiographic and symptomatic improvement of pneumonitis, and he proceeded to surgery.
The patient experienced an atypically rapid and large-volume lung reaction at doses that do not usually produce such a response. In addition, the patient experienced a complete response to neoadjuvant CRT. Pathologic complete response rates for locally advanced lung cancer patients undergoing trimodality therapy are ~15% to 20% and result in improved overall survival when compared to incomplete responders.15,16 The patient had no known family or personal history of syndromes associated with increased radiosensitivity; however, the marked response of both normal and neoplastic tissue to RT should be noted. The literature does not address pneumonitis as a predictor of either radiographic or pathologic response to neoadjuvant CRT.
CONCLUSION
We report a patient with locally advanced lung cancer who demonstrated an atypically rapid and dramatic lung tissue radiographic response to neoadjuvant CRT while showing only mild-to-moderate clinical manifestations of radiation pneumonitis. His functional parameters declined sufficiently that he was no longer a surgical candidate. A limited course of oral corticosteroids resulted in sufficient improvement to make him again eligible for surgery. We also observed a complete pathologic response after definitive resection, which may reflect enhanced radiosensitivity of both normal and neoplastic tissue. Radiation pneumonitis, although well documented, continues to be poorly understood and difficult to predict, but may prove useful in predicting clinical responses to neoadjuvant therapy in locally advanced lung cancer.
REFERENCES
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452-1460.
- Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction,and prevention. Int J Radiat Oncol Biol Phys. 2005;63:5-24.
- Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Bio. Phys. 2005;61:318-328.4. Movsas B, Raffin TA, Epstein AH, Link CJ Jr. Pulmonary radiation injury. Chest. 1997;111:1061-1076.
- Choi YW, Munden RF, Erasmus JJ, et al. Effects of radiation therapy on the lung: radiologic appearances and differential diagnosis. RadioGraphics. 2004;24:985-998.
- Marks L, Bentzen S, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76:570-576.
- Tsujino K, Hirota S, Endo M, et al. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2003;55:110-115.
- Kim M, Lee J, Ha B, Lee R, Lee KJ, Suh H. Factors predicting radiation pneumonitis in locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;29:181-190.
- Chen YM, Shih JF, Perng RP, Tsai CM, Whang-Peng J. A randomized trial of different docetaxol schedules in non-small cell lung cancer patients who failed previous platinum-based therapy. Chest. 2006;129:1031-1038.
- Jin H, Tucker SL, Hui HH, et al. Dose-volume thresholds and smoking status for the risk of treatment related pneumonitis in inoperable non-small cell lung cancer treated with definitive radiotherapy. Int J Radiat Oncol Bio Phys. 2009;91:427-432.
- Rodrigues G, Lock M, D’Souza D, Yu E, Van Dyk J. Prediction of radiation pneumonitis by dose–volume histogram parameters in lung cancer—a systematic review. Radiother Oncol. 2004;71: 127-138.
- Kong F, Hayman J, Griffith K, et al. Final toxicity results of a radation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors of radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;45: 1075-1086.
- Graham M, Purdy J, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1999;45:323-329.
- Magaña E, Crowell R. Radiation pneumonitis successfully treated with inhaled corticosteroids. South Med J. 2003;96:521-524.
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379-386.
- Albain K, Rusch V, Crowley J, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA(N2) and TIIB non-small-cell lung cancer: mature results of Southwest Oncology Group Phase II Study 8805. J Clin Oncol. 1995;13:1880-1892.
