Proton therapy – What is it and what can it do to help my patients?
Images
The birth of proton beam therapy could be designated as the publication, by Robert R. Wilson in his 1946 manuscript, of the concept of using the unique advantages relative to photons.1 Twelve years later, a team from the Lawrence Berkeley National Laboratory in California published the first series of human treatments in 1958.2 Many other forms of particle therapy, such as anti-proton, neon, carbon, oxygen, and neutron, have also been studied.3-7 The most common type of particle therapy in use is electron therapy. It has far less mass than other particles and is not the subject of this essay because its lack of mass makes it unable to share in the dramatic physical advantage that heavier charged particles can demonstrate in the clinic; ie, very rapid stopping of the beam.
Rather, this review article focuses on proton therapy, currently the most common, clinically used heavy charged particle therapy worldwide. Indeed, it is now available to a large portion of patients being considered for advanced radiation treatment techniques.
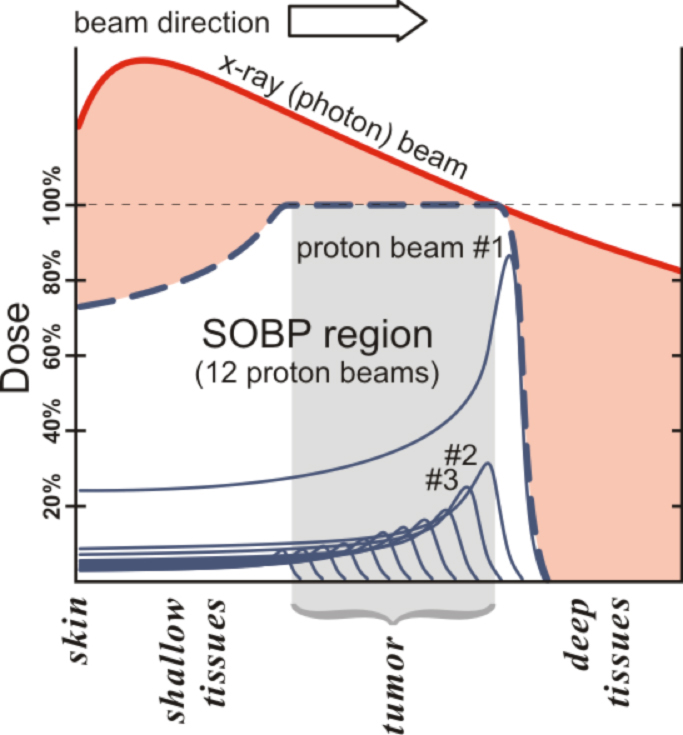
The critical aspect of proton beam therapy—all heavy charged particles, really—that interests clinicians so greatly is its pronounced physical property known as the Bragg peak (Figure 1). The use of proton beams in the clinic is typically made up of a sum of multiple, single 2-mm- to 3-mm-thick pristine beams, or Bragg peaks. In Figure 1, 12 such peaks are shown to make one broader peak called a spread-out Bragg peak (SOBP). The shape of the SOBP is tailored to mimic the shape and location of a tumor as outlined by the treating physician. No clinician today would use a single x-ray beam to treat a tumor, so the demonstrated beam comparison in Figure 1 is an oversimplification of how things are actually done in the clinic for photons (conventional x-rays). Proton beam therapy is used because abruptly stopping the beam in a controllable fashion allows significant avoidance of normal structures while delivering high doses of therapy to tumors. Proton therapy is also used because its biology is similar, in dosimetric terms, to photons, with the relative biologic effect (RBE) felt to be about 1.1 relative to cobalt dose, making dosimetry use simpler than the higher RBE neutron, which lacks the Bragg peak.8
The simplest type of common tumor treated by both protons and photons is prostate cancer. In current prostate cancer therapy, lateral proton beams are typically used and multiple (usually 5 to 9) photon beams are used with intensity-modulated radiation therapy (IMRT) via a multi-leaf collimator (MLC). Thus, one typically delivers half the dose via one beam in protons and 20% or less dose in photons. Because of this, the relatively high entry doses with photons, such as those shown in Figure 1, are mitigated because only 20% of a given dose is required at the depth of the tumor. However, the exit dose issue for photons remains; and integral dose, as a result of this necessary method to optimize photons, is almost always higher for photons than for protons. Multiple examples can be found in the literature comparing the dosimetry of protons and photons for multiple types of tumors in all age ranges of patients.9-17 Proton beam therapy’s ability to spare normal tissue is for the most part superior to IMRT, but it is not that simple because we are still learning when and how much normal tissue needs to be spared.
The use of proton beam therapy in the clinic is still relatively new; currently, it is being used primarily as a substitute for photon therapy at the same doses and fractionation schedules that photons would be used, but with improved normal tissue sparing. This is because we lack data suggesting that doing otherwise is superior.
Rationale for proton therapy in adults
In adults, proton beam therapy is governed by clinical situations where a tumor requiring a high dose lies adjacent to a normal structure that cannot tolerate the resultant dose from the best dose gradient IMRT without a very high risk for damage. Proton beam therapy is commonly used for tumors of the base of the skull,18 spine,19 pelvis, brain, and for recurrent disease in which all nearby tissue has already received maximal dose but more radiation must be delivered to the same tissues at a significant dose.
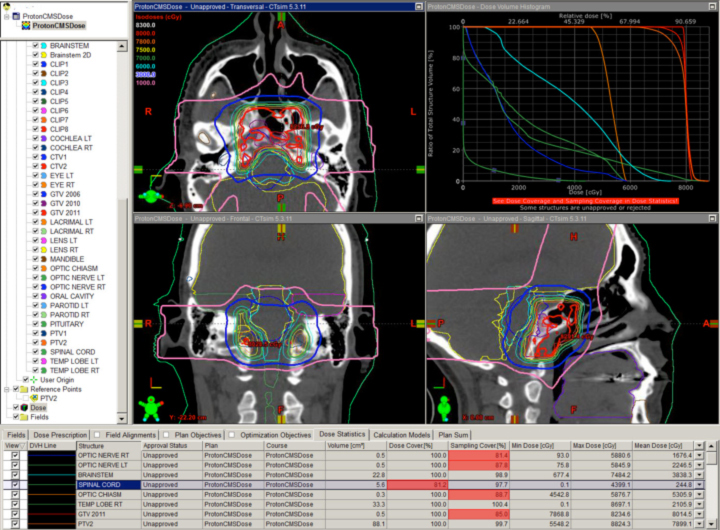
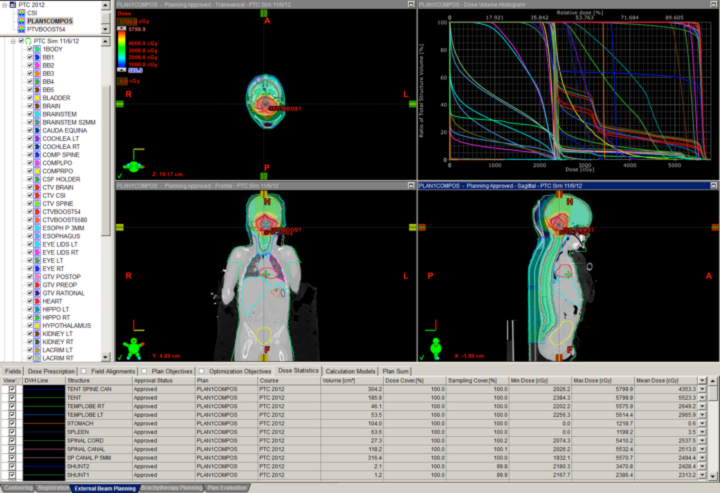
The classic example is clival chordoma (Figure 2). In these cases, the dose gradient has to fall from 78 Gy to 63 Gy or lower in several millimeters to protect the brainstem and the optic apparatus simultaneously. Nothing except charged-particle therapy allows this to happen in this specific anatomic and clinical context while also keeping safe the many cranial nerves in the region. The ability to keep the dose to the brainstem and optic nerves to a reasonable level is shown as well. This could not be achieved with photons.
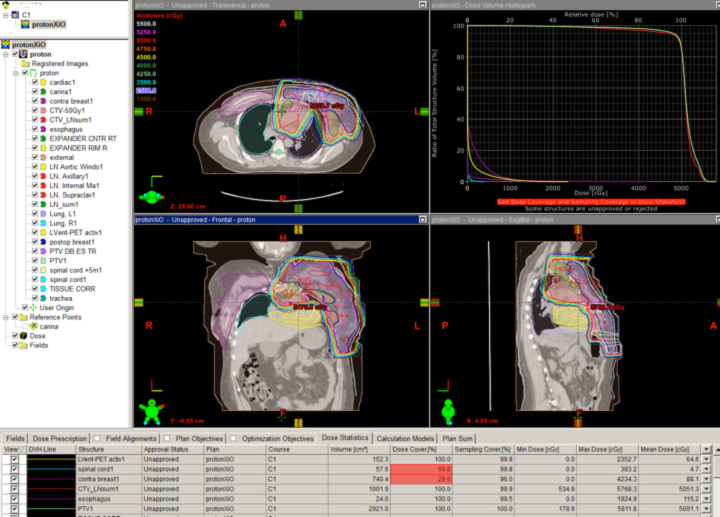
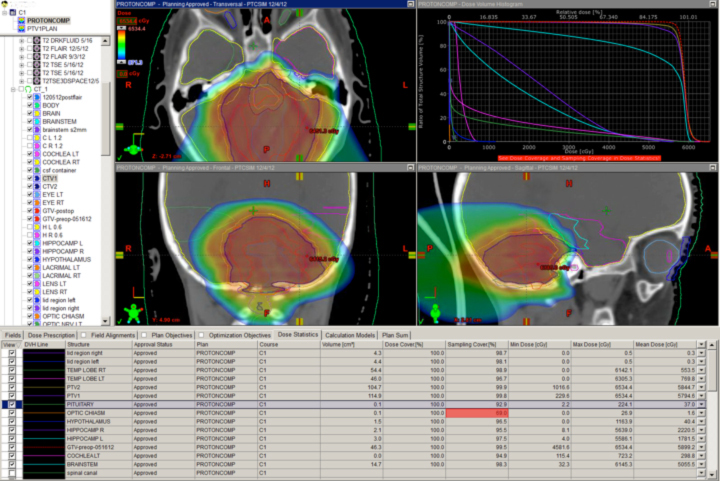
A novel indication for proton therapy in adults perhaps may be breast cancer. When patients present with complex chest wall tumors with positive lymph node findings, photons often are unable to spare the heart and lungs. In a post-mastectomy case where the supraclavicular, axillary, internal mammary, and aortic window nodes were positive, a two-field proton chest wall plan was developed that spared the lung, heart, and esophagus (Figure 3).
No photon plan could be achieved to do the same without significant lung and cardiac doses. New data suggest cardiac radiation is associated with severe late toxicity in breast cancer patients, so the proton plan was far superior from this aspect. This kind of proton plan may allow the patient to receive higher doses of drugs toxic to the heart, to avoid otherwise unavoidable late effects of standard doses to the heart, or subsequently to receive more radiation near the heart without exceeding normal tissue tolerance.
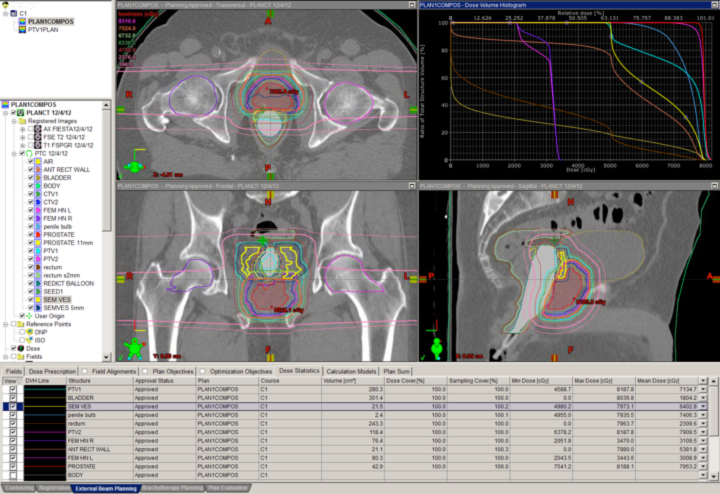
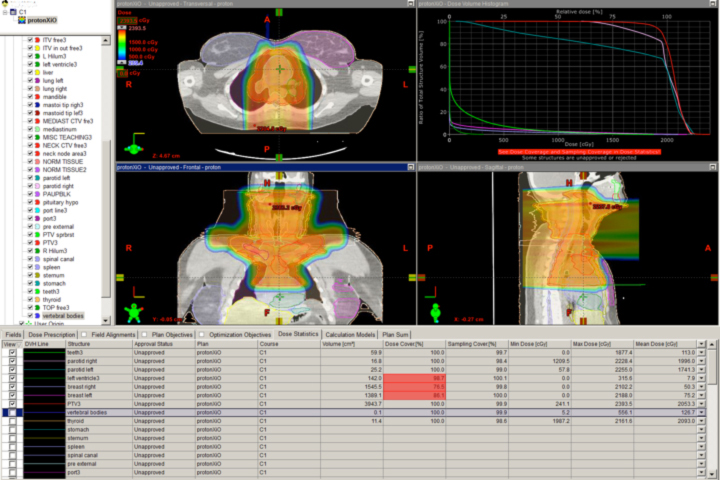
Many clinicians believe the anatomic issues of prostate cancer justify the use of advanced technology, and these same issues make proton therapy a logical choice. Figure 4 depicts a typical prostate proton plan for reference. Most radiation oncologists will note that the posterior rectum and anterior bladder doses are very low as a result of the beams used (laterals). Some proton centers use one beam per day, alternating sides from day to day. Others deliver beam to both sides every day. Immobilization for proton therapy is different than for photon therapy because protons are more sensitive to small distance variations. At the Indiana University Health Proton Therapy Center (IUHPTC), patient immobilization includes a daily rectal balloon placement and a customized body mold device (similar to what is used in spinal surgery patients) to keep the patient’s skin-to-prostate distance stable day to day.
Rationale for proton therapy in children
The same rationale used in adults holds for children, with the additional concern for total volume of dosimetric exposure and avoidance of secondary toxicities.20,21 Data show that growing tissues are more likely to experience damage from radiation. To reflect this, pediatric dose tolerance is lower than what is used in adults. In addition, the total number of years before a child is likely to experience side effects is greater than that of an adult simply because the child may very well live long enough to have a side effect, while an adult may not. As a result, the general consensus in the specialty is that children are very well served by proton therapy; and that in general it is worth considering whether normal tissue can be spared to a significant degree. Early modeling and retrospective data reviews suggest decreased secondary malignancy rates and decreased toxicity.22-26 Prospective studies are ongoing and most trials in the Children’s Oncology Group (COG) currently allow proton therapy. Recently published data suggest that protons may lower in-field second malignancy rates by a factor of 2 to 10.27
The classic case that justifies proton therapy in a child is craniospinal irradiation (CSI) for medulloblastoma. Data presented within the last year suggest that the risk of secondary cancer is about 20% or lower with protons relative to photons. These data also suggest that protons have a lifetime secondary cancer risk of 7.7% in passive scattered form, while photons had a 93% lifetime risk for a young child with standard-risk medulloblastoma.28 Supine proton CSI with dose stopping before the thyroid, breast, lungs, esophagus, heart, gut, and bladder is shown in Figure 5.29
Brain tumors in children also benefit from proton therapy. Figure 6 shows a typical fourth ventricular ependymoma case highlighting protons’ ability to spare the cochleae, hippocampi, optic apparatus, and hypothalamus. Avoiding even low to moderate doses to these critical organs is impossible with IMRT or conventional radiation therapy.
Proton therapy also enables clinicians to treat tissues in the torso and yet spare patients from second malignancies not currently thought to be traditional proton cases. Figure 7 shows a case of Hodgkin’s disease where breast tissue was spared on a local protocol. The next Children’s Oncology Group (COG) study for Hodgkin’s disease may allow proton therapy.
New technologies that are coming into focus
Proton beam therapy is expensive and cumbersome. The development of newer centers comes with evolutionary improvement and simplification of beam production and shaping. Cyclotrons requiring large staff in older centers are being replaced by simplified devices that demand fewer staff. Beam stability and energy are being improved so that deeper tumor targets can be treated. Purchase price is falling via newer, simpler designs. Finally, patient-specific devices (PSDs) analogous to conventional edge and transmission blocks may be eliminated by so-called “pencil-beam” scanning systems.
This last item is perhaps the most clinically interesting and important development in proton therapy. While the physics and engineering of these devices is beyond the scope of this article, pencil beam proton therapy renders obsolete the use of metal apertures to shape the beam edge and Lucite compensators to shape the beam’s distal range. The closest analogy in photon therapy is using the blocks in 3-dimensional (3D) conformal therapy with dynamic MLC-driven therapy. Pencil-beam proton therapy is truly a 3-dimensional dynamic form of intensity modulation. In addition, the proximal beam edge can be modulated to make proximal dose avoidance a reality. In theory, this technology requires fewer beams and far less hardware to deliver proton therapy than at present. These two features suggest that a cost savings, on top of superior clinical dosimetry, is achievable.
Pencil beam proton nozzle technology is in its infancy and the penumbra achievable with the best devices is not yet as good as that achieved with apertures and compensators, but the lack of metal and material interaction, in theory, lowers the generation of neutrons, which is associated with the smaller but real risk of secondary cancers resulting from proton therapy. The next generation of these devices will likely radically improve proton therapy. It is an active area of research around the world.
Controversies
The primary controversies in proton beam therapy are financially related: Who gets therapy and how to pay for expensive new centers. The cost of proton therapy limits patient access to proton therapy centers. The higher financial cost makes treatment for diseases treated well with conventional therapy controversial if treated with proton therapy, even with an established but small incremental clinical benefit. By far, the most controversy to date for proton therapy has resulted from a recent review of prostate cancer therapy. This has taken place in the context of a paradigm shift for prostate cancer as a whole, and with prostate cancer treatment comprising one of the largest sources of revenue for any radiation center, proton- or photon-based. The most recent and most robust research, published by a group at Yale, suggests that IMRT for prostate cancer may be nearly equally effective clinically as the more expensive proton therapy in men aged 66 and over. 30 If the two therapies cost exactly the same, the issue would be far less controversial. The study has not followed patients long enough to make any long-term conclusions as of yet with 12-months follow-up being reported, so it is unclear if this is a true statement long term. As costs come down, the use of proton and other particle therapies will become less controversial.
Limitations of proton therapy
At present, protons have concrete limitations relative to photons that clinicians must understand to best take advantage of the treatment. First, proton therapy generally takes much longer than photon therapy to go from simulation to beam delivery. It is not currently best used for emergent radiation therapy because of this decreased nimbleness.
Second, proton therapy does not have widespread availability, so patients may not hear about it from referral physicians even when it would be superior to conventional therapy. Travel and housing costs can make the technology prohibitive even when patients and physicians want to employ protons.
Third, protons are inherently more complex than photons, and problems can be more difficult to fix on a daily basis. This means that staffing is more crucial for these centers than for photon-based centers. Physics staffing is likely 2 or 3 times that of image-guided photon therapy centers. The learning curve for protons is also steeper and the chance to learn how to do proton therapy is more limited, also making staffing more difficult for proton therapy.
Fourth, proton therapy requires the treatment team to have the appropriate expertise to treat the mix of tumors for which it is best employed: complex tumors next to the brain and spine in adults, children, and heavily pretreated patients. As the number of proton centers increases, the best ways to exploit it will be more widely understood and taught.
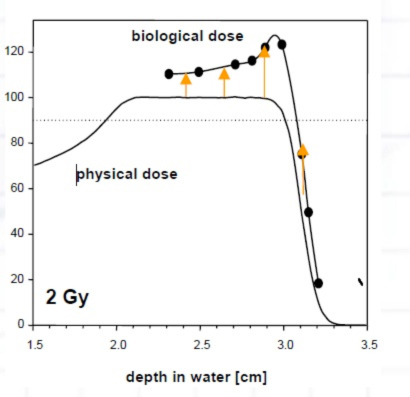
Fifth, the dosimetry of the proton beam, in terms of biology, is not linear, and methods exist for mitigation of the increased biologic dose at the end of the Bragg peak that are often not obvious to those who don’t routinely practice proton therapy (Figure 8). Careful attention to beam selection, beam stopping location, and the number of beams is needed to prevent unexpected adverse biologic outcomes.
Finally, because proton therapy is so sensitive to distance from patient surface to the stopping point, it is far easier to have a geographic miss while using protons than it is while using photons. To correct for this, patients need to be assessed frequently and perhaps imaged with on-treatment, high-quality CT or MRI scans to assess changes. Departments using protons must be able to respond to range changes quickly for patients at risk for this issue. The development of photon-adaptive radiotherapy directly impacts this aspect of proton treatment because the identical issues must be addressed.31
Conclusions
Proton beam therapy is a clinically relevant, accepted form of radiation therapy that is likely to endure and is justified by current data. New technologies are emerging to improve treatment delivery. Proton beam therapy is benefiting indirectly from the progress made on all fronts of medicine to deliver better therapy to patients using better technology in planning and imaging. As cost decreases and availability increases, the technology will become more pervasive and data will be developed to further specify where and when it is best used.
Time will tell if we are able to lower the costs enough to make the technology more routine and less expensive for patients. Currently, protons are appropriate for first-line consideration in many pediatric, spinal, base-of-skull, head and neck, pelvic, and retreatment tumors. They may also prove superior for some subgroups of lung, breast, and prostate patients.
To be sure, proton therapy is no more effective, in many cases, than conventional therapy; due to geographic and other issues, it may even pose a greater hardship for some patients. Discussing cases with proton therapy centers will allow referral physicians to better establish options for their patients.
However, proton therapy is here to stay, and many clinical trials are under way to better understand how and when to use it. Keeping abreast of this technology will be exciting for all radiation oncologists, as the promise to treat cancer patients with fewer side effects is something for which we all strive.
References
- Wilson RR. Radiological use of fast protons. Radiology. 1946.
- Lawrence JH, Tobias CA, Born JL. Pituitary irradiation with high-energy proton beams: A preliminary report. Cancer Res.1958;18:121-134.
- McDonald MW, Fitzek MM. Proton therapy. Curr Probl Cancer. 2010;34:257-296.
- Halperin EC. Particle therapy and treatment of cancer. Lancet Oncol. 2006;7:676-685.
- Bassler N, Alsner J, Beyer G, et al. Antiproton radiotherapy. Radiother Oncol. 2008;86:14-19.
- Boone ML, Lawrence JH, Connor WG, et al. Introduction to the use of protons and heavy ions in radiation therapy: historical perspective. Int J Radiat Oncol Biol Phys. 1977;3:65-69.
- Ando K, Kase Y. Biological characteristics of carbon-ion therapy. Int J Radiat Biol. 2009;85:715-728.
- Jones B, Dale RG, Carabe-Fernandez A. Charged particle therapy for cancer: The inheritance of the Cavendish scientists? Appl Radiat Isot. 2009;67:371-377.
- Kozak KR, Adams J, Krejcarek SJ, et al. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys. 2009;74:179-186.
- Moon SH, Shin KH, Kim TH, et al. Dosimetric comparison of four different external beam partial breast irradiation techniques: Three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, helical tomotherapy, and proton beam therapy. Radiother Oncol. 2009;90:66-73.
- Torres MA, Chang EL, Mahajan A, et al. Optimal treatment planning for skull base chordoma: Photons, protons, or a combination of both? Int J Radiat Oncol Biol Phys. 2009;74:1033-1039.
- Kosaki K, Ecker S, Habermehl D, et al. Comparison of intensity modulated radiotherapy (IMRT) with intensity modulated particle therapy (IMPT) using fixed beams or an ion gantry for the treatment of patients with skull base meningiomas. Radiat Oncol.2012;7:44.
- Oelfke U, Bortfeld T. Optimization of physical dose distributions with hadron beams: Comparing photon IMRT with IMPT. Technol Cancer Res Treat. 2003;2:401-412.
- Chera BS, Rodriguez C, Morris CG, et al. Dosimetric comparison of three different involved nodal irradiation techniques for stage II Hodgkin’s lymphoma patients: Conventional radiotherapy, intensity-modulated radiotherapy, and three-dimensional proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:1173-1180.
- Song WY, Huh SN, Liang Y, et al. Dosimetric comparison study between intensity modulated radiation therapy and three-dimensional conformal proton therapy for pelvic bone marrow sparing in the treatment of cervical cancer. J Appl Clin Med Phys. 2010;11:3255.
- Howell RM, Giebeler A, Koontz-Raisig W, et al. Comparison of therapeutic dosimetric data from passively scattered proton and photon craniospinal irradiations for medulloblastoma. Radiat Oncol. 2012;7:116.
- Milby AB, Both S, Ingram M, et al. Dosimetric comparison of combined intensity-modulated radiotherapy (IMRT) and proton therapy versus IMRT alone for pelvic and para-aortic radiotherapy in gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2012;82:e477-484.
- Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175 Suppl 2:57-63.
- Park L, Delaney TF, Liebsch NJ, et al. Sacral chordomas: Impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys. 2006;65:1514-1521.
- Merchant TE. Proton beam therapy in pediatric oncology. Cancer J. 2009;15:298-305.
- Wilson VC, McDonough J, Tochner Z. Proton beam irradiation in pediatric oncology: An overview. J Pediatr Hematol Oncol.2005;27:444-448.
- Merchant TE, Hua CH, Shukla H, et al. Proton versus photon radiotherapy for common pediatric brain tumors: Comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51:110-117.
- Hattangadi JA, Rombi B, Yock TI, et al. Proton radiotherapy for high-risk pediatric neuroblastoma: Early outcomes and dose comparison. Int J Radiat Oncol Biol Phys. 2012;83:1015-1022.
- Hoch BL, Nielsen GP, Liebsch NJ, et al. Base of skull chordomas in children and adolescents: A clinicopathologic study of 73 cases. Am J Surg Pathol. 2006;30:811-818.
- Miralbell R, Lomax A, Cella L, et al. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54:824-829.
- Chung CS, Keating N, Yock TI, et al. Comparitive analysis of second malignancy risk in patients treated with proton therapy versus conventional photon therapy. Int J Radiat Biol Phys. 2008;72: .
- Paganetti H, Athar BS, Moteabbed M, et al. Assessment of radiation-induced second cancer risks in proton therapy and IMRT for organs inside the primary radiation field. Phys Med Biol 2012;57:6047-6061.
- Zhang R, Howell RM, Giebeler A, et al. Comparison of risk of radiogenic second cancer following photon and proton craniospinal irradiation for a pediatric medulloblastoma patient. Phys Med Biol. 2013;58:807-823.
- Buchsbaum JC, Besemer A, Simmons J, et al. Supine proton beam craniospinal radiotherapy using a novel tabletop adapter. Med Dosim. 2012 Aug 27. [Epub ahead of print]
- Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: Patterns of care and early toxicity. J Natl Cancer Inst. 2013;105:25-32.
- Simone CB, 2nd, Ly D, Dan TD, et al. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother Oncol. 2011;101:376-382.
- Filipak M. Thematic diagram showing dose as a function of depth for overlay of proton radiotherapy and x-ray radiotherapy to facilitate a comparison of the two radiotherapy methods. Wikipedia; 2012.33. IUHPTC. Treatment Plan Image completed in CMS XIO and/or Varian Eclipse at the IU Health Proton Therapy Center in accordance with IRB policy allowing de-identified images. 2013. Plans are shown with dosimetry isodose lines and/or colorwash and DVH data.
- Cheng CW, Das IJ, Srivastava SP, et al. Dosimetric comparison between proton and photon beams in the moving gap region in cranio-spinal irradiation (CSI). Acta Oncol. 2012 May 4.
- Singhal M, Vincent A, Simoneaux V, et al. Overcoming the learning curve in supine pediatric proton craniospinal irradiation. J Am Coll Radiol 2012;9:285-287.
- Wallace WH, Moell C, Kremer LCM. The European Symposium on Late Complications after Childhood Cancer 2011. ESLCCC 2011. Amsterdam; 2011.
