Stereotactic body radiation therapy (SBRT) for early-stage primary liver cancer (HCC)
Images
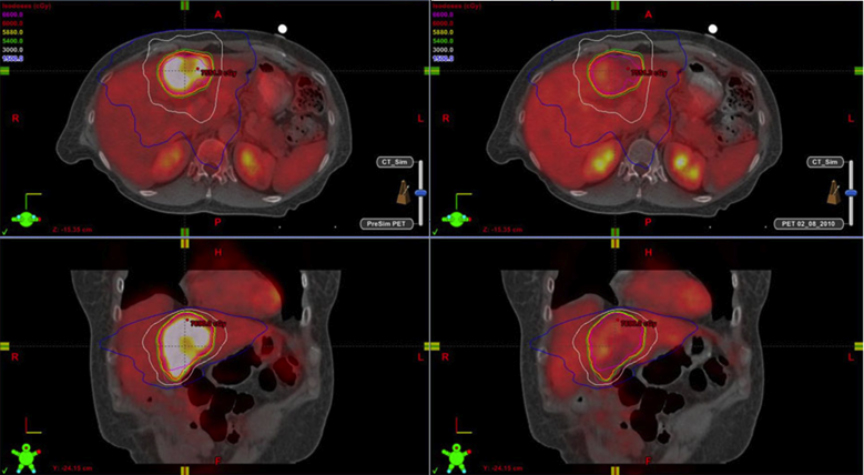
Hepatocellular carcinoma (HCC) is the sixth leading cause of cancer globally (fifth in men and eighth in women) with 750,000 new cases per year.1 Its global prognosis is very poor with only a 7% 5-year survival.1 Because of such a poor survival rate, at 696,000deaths, it is currently the third leading cause of cancer mortality after lung and stomach cancer. Within 5 years, global HCC mortality is expected to be second only to lung cancer.1
In the United States (U.S.), while primary HCC is small at ~20,000 cases, it is one of the few U.S.-based cancers whose death rate is rising,primarily due an increase in hepatitis C and obesity induced nonalcoholic steatohepatitis (NASH).3 U.S. 5-year survival rates have improved only marginally in the past 40 years, from 4% in the early 70s to 14% currently,2 second only to pancreatic cancer, which has a 6% survival rate.
Eighty percent of HCC cases arise in developing countries and over 55% of all HCC cases are found in mainland China.1 Asia has a high incidence of chronic viral hepatitis infection (hepatitis B or C), which contributes to the high rate of HCC. Liver cirrhosis, a late-term effect of hepatitis infection, results in a 10-fold risk in HCC, hence the high Asian risk factor.1 Like lung cancer, HCC is a silent disease whose effects typically do not show up until late stage presentation. Early-stage diagnosis gives a poor 5-year survival of 26%, whereas late-stage diagnosis gives a miserable 2% survival.2
Invasive therapies
Invasive therapies that can improve on the 5-year survival of HCC include resection and transplantation. Their improvement in 5-year survival rates range from 30% to 50% and 60% to 70%, respectively. These surgical techniques, first developed in 1949 and 1967, are unfortunately eligible to < 30% of HCC patients due to multiple factors, including tumor size and location within the liver, vascular invasion,and poor liver function.4 Further, in several large key Asian societies, transplantation is neither culturally acceptable nor clinically practical.5 Some less invasive treatments include: percutaneous ethanol injection6(PEI), transarterial chemo embolization7(TACE) radiofrequency ablation8(RFA) and yttrium-90 brachytherapy,9 but these are either only palliative or suffer from many of the same eligibility contraindications as surgery.
Treatment challenges
Treating the liver for HCC using any technique is a challenge for two reasons. Firstly, it is two diseases in one: a chronic viral liver disease and a malignancy resulting from that chronic liver destruction. Secondarily, the heterogeneity (etiology and prognosis) of those different diseases affects treatment and survival.
Tumor stage and underlying liver function are both major determinants of the treatment selection as well as prognosis in HCC patients,thus allowing no more than a 20% chance for potentially curative therapies.9The accurate assessment of disease differential, disease extent,and liver function significantly impacts the choice and targeting of treatment. Key to the safe treatment of liver disease is the preservation of liver function.9
Lessons learned from surgery provide a model for treating with radiation. In surgery, assessment is done using a variety of metrics to assess liver function and being cognizant of liver volume treated. Assessment metrics include: Child-Pugh (CP) score10—a liver function classification system, A to C, scoring severity of disease, CT perfusion, Model for End-Stage Liver Disease (MELD) and Barcelona Clinic LiverCancer (BCLC) staging.11 In liver surgery for HCC, preservation of function dictates resections of no more than 0.5% of body weight and45% of liver volume (~450cc) and resections are only performed when a variety of liver function measures, such as a CP score of no worse than class A, are met. The relationship between CP liver function and BCLC staging is nicely detailed in a recent review paper from Korea.12
SBRT for HCC: Early efforts and rationale
The liver was first a target for radiation therapy as early as 1924. The use of conventional external beam radiation therapy (RT) as a curative technique, was hampered by early evidence of radiation’s severe toxicity to the diseased liver defined as radiation-induced liver disease(RILD).10,13 RILD manifests in long-term migration of Child-Pugh score from A to B to C, resulting in likely liver failure with the later scores. A decline in liver function is more likely in patients with higher baseline CP scores and in those with advanced disease requiring larger volumes of irradiated liver.14
Thus all forms of RT for HCC have been slower to evolve due to the liver’s low tolerance to RT, further reduced in the cirrhotic liver. This is especially true where high doses of radiation are distributed throughout the liver as is the case with non image-guided, non-IMRT treatments. Palliative liver radiation has recently been shown to improve quality of life in patients with active symptoms from HCC or liver metastases.15
Liver SBRT, the precise delivery of potent doses of radiation in a small number of fractions to the liver, was first performed by Blomgren &Lax in 1995 in 1 to 3 fractions of 20 to 45 Gy.16 SBRT is the non-CNS extracranial extension of stereotactic radiosurgery (SRS), which neurosurgeons have been using for ablation of tumors in the brain and spine for >30 and >10 years, respectively. For primary CNS tumors, the intent is primarily curative. With very high dose rates per session, SRS and SBRT treatments require precision with tight margins to the tumor and minimum dose to surrounding organs at risk and normal tissues and employ an overwhelmingly ablative radiobiological mechanism.This is in contrast to conventional RT treatments where cells are allowed to repopulate and precision requirements are an order of magnitude less stringent.
The advantage of refined SBRT techniques is that they allow for a safer administration of higher levels of dose while minimizing the potential of RILD.17 The past decade has seen a small number of single institution SBRT liver trials every couple of years on various platforms(Figure 1). Klein pointed out that there has been a doubling to 600 publications, on the use of radiation to treat liver tumors, in the 5-year period “2005-2010,” over the prior 5-year period.11 Furthermore, over 75% of all SBRT for HCC studies have published in the last 6 years, and over 66% published in the past 3 years.18-36
Refinements in SBRT HCC treatments have led to substantially improved results over both RT and those early SBRT treatments, which had some grade 5 toxicities. Liver toxicity with modern SBRT techniques is low because of precise stereotactic targeting and that ablative dose volumes are substantially reduced. Liver toxicity is uncommon in SBRT treatments where the effective volume irradiated is < 30% of total liver volume and where > 800 cc gets < 18 Gy as shown by a recent Korean paper.18
SBRT for HCC: Recent studies
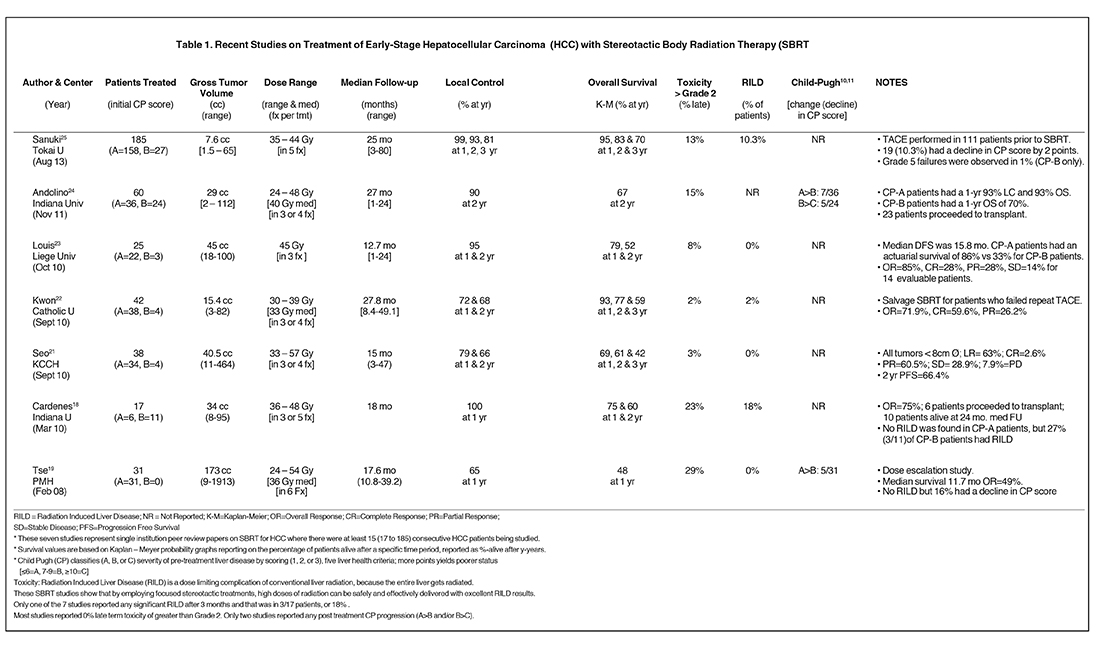
A number of centers have recently reported on SBRT-based HCC treatment and several are summarized in Table 1.
- A Toronto group reported on a phase I liver cancer dose-escalation trial, which included 31 HCC patients with an average gross tumor volume (GTV) of 173 cc [9 to 1913 cc].19,35 With a median dose 36 Gy (24 to 54 Gy) in 6 fractions, they achieved a 12-month local control (LC) rate of 65%. For patients with portal vein thrombosis (PVT), median survival was 11.6 months, which improved to 17.2 months for patients with no PVT. Overall survival (OS) was 48% at 1-year and 16% (5/31) of patients had a poorer level of liver function measured by a decline to in CP score. No patients had RILD and this study set the stage for other SBRT studies, as previously RILD was considered a treatment limiting toxicity of conventional radiation treatments.
- A group from Indiana reported on 17 patients with 1 to 3 targets (25 HCC total).20 They had a dose escalation trial delivering 36 to 48 Gy infractionation schedules from 5x8 Gy to 3x16 Gy. Their tumors (cumulative diameter ≤ 6 cm) were much smaller with an average GTV of 34 cc.With a mean follow up of 18 months, they achieved 100% LC at 1-year and an OS of 75% and 60% at 1 and 2 years respectively. Three patients had grade 3 to 4 toxicity. RILD was not observed in any CP-A patients, but was observed in 27% of CP-B patients.
- One Korean group documented a prospective registry of 38 patients with tumors < 10 cc treated in 33 to 57 Gy in 3 to 4 fractions.21One and 2-year LC was 79 and 66% and 1-, 2- and 3-year OS was 68%, 61% and 41%, respectively. Only one grade-3 skin toxicity was reported.For those 26 patients who received a dose of > 42 Gy, a 2-year OS was reached.
- Another Korean group22documented 42 HCC patients with a median GTV of 15.4 cc, treated with a median dose of 33 Gy (30 to 39 Gy) in3 fractions. With a median follow up of 27.8 months, they achieved a LC of 72 and 67.5% at 1 and 2 years respectively and OS of 92.9, 77.3 and58.6% at 1, 2 and 3 years respectively. Consistent with other SBRT studies, they had very low toxicity (< 2 %) and low incidence of radiation induced liver disease RILD (2%).
- A Belgian group23reported on 25 HCC patients treated with a median dose of 45 Gy in 3 sessions. The treatment was well tolerated overall, and there were no grade 4 toxicities. Overall, actuarial survival was 79% and 52% at 1 and 2 years with a mean overall follow up of 12.7months. CP-B patients had a 33% actuarial survival versus CP-A patients at 86%. No RILD was observed and excellent response to treatment was observed with overall response of 85% in 14 evaluable patients.
- In the largest North American study to date, a paper24 from the Indiana group20 reported on 60 patients treated with an average GTV of 29 cc. 36 CP-A patients were treated with 30 to 48 Gy in 3 fractions. With a median follow- up of 27 months, they achieved a 1-year LC of 93%and a 1-, 2- and 3-year OS of 93%, 77% and 70%, respectively. . 19% had a decline in CP score and 2 patients had grade 5 toxicities. The same paper further reports on 24 patients with CP-B scores treated with 24 to 48 Gy in 3 fractions whose 1-, 2- and 3-year OS were reduced to 70%, 50% and 50%, respectively. Of the 60 patients, 23 went on to othotopic liver transplant (OLT).
- Several even larger studies have more recently been reported from across Asia.25-27 The largest single study to date is from Japan.25 In this study, 221 patients with 237 single small HCC lesions were treated from 2005 to 2012. Of these, 185 met a variety of clinical criteria and were evaluable in this study. Patients were treated with either 35 Gy (48 pts) or 40 Gy (137 pts) depending on the CP scores and other factors. The 3-year local control and overall survival rates were 91% and 70% respectively. Ten local recurrences were observed at a median of 21 months. The dosing schemes provided equivalent results, acute toxicities (> grade 2) were observed in only 13% of patients and the procedure was deemed to provide excellent and safe outcomes.
Functional imaging techniques may be able to prospectively predict SBRT tumor control. A group from Taiwan retrospectively assessed31 HCC patients (41 tumors) who had 18F-FDG PET prior to SBRT.28 They determined that a TSUVmax (maximum standardized uptake value of the tumor) cutoff value of 3.2 was a good prognostic indicator of tumor control for patients treated with SBRT. They concluded that 18FFDG PET may help in patient selection and dose adjustment for HCC candidates for SBRT.
Definitive liver surgery (transplantation or resection) is considered the only curative option for HCC.31 SBRT as a bridge to transplant,where the patient is a candidate for OLT, is more common in North America, with a number of centers taking that approach.24,32-34 A pilot study from the Toronto group was the first paper in the surgical literature.32 They reported on 5 of 10 patients treated with SBRT who successfully underwent OLT and are cancer free. A group from New York33 treated 27 HCC patients with SBRT. Seventeen of these had OLTallowing for explants tissue analysis and evaluation. Thirty-seven percent had complete or partial response on imaging, and 93% were stable or had at least partial response. Of 22 pathologically evaluated lesions, 37% had total or partial response to SBRT. More recently, a group from Texas34 reported on the long-term outcomes of SBRT as a bridge to transplantation with a median follow-up of 62 months from the time of SBRT. Ten patients with 11 HCCs were treated and transplanted. All 10 are alive and free of disease with a 5-year overall survival and disease free survival of 100%. Surgical candidates who fail, or are unsuitable for other treatments, and have a high risk of for disease progression, which would lead to being delisted, could be well served with SBRT as a bridge to transplant.
Conclusion
In summary, small HCC tumors appear to be good candidates for SBRT, though larger (over 1000 cc) tumors have been successfully treated as well.19,29,30 Risk adaptation and individualization must be used to avoid serious toxicities seen in early treatments. Table 1 shows 7recent studies, with at least 15 patients, having excellent outcomes for HCC. With one exception, Grade 3 or higher toxicities were 15% or less. The most recent 6 studies have a minimum 2-year survival of over 50% with an average 2-year survival of 75%. Three-year survival isas high as 70% for the CP-A subset of patients.24,25 Due to the variability of utilization of SBRT in the course of HCC treatment at centers,overall survival from the conclusion of radiation is not always the ideal metric to judge the success of the treatment. Normal tissues will limit doses that can be safely delivered. Treatment beam modulation and image-guidance technologies, which can reduce PTV will aid in successful HCC treatment and OAR avoidance. With the development of modern sophisticated radiotherapy machines, increasing use of SBRT for HCC is expected. Combination therapies are expected to be of additional help. These results provide a strong argument for randomly controlled phase I/II trials.35 An NCI funded, phase III trial RTOG 1112: Sorafenib versus SBRT followed by Sorafenib, whose goal is to determine if SBRT can help extend HCC survival especially for later stage disease,14,35 opened in January 2013. Treatments with some of the latest radiosurgery devices now allow for precise delivery of the high doses required by SBRT with beam-on times of under 5 minutes as described in the paper by Mancosu.36
References
- World Cancer Report 2008. Boyle P & Levin P eds. World Health Organization: International Agency for Research on Cancer; Lyon, France http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/wcr_2008.pdf.
- Global Cancer Fact & Figures, 2nd Edition, Atlanta, American Cancer Society, 2011.
- Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Growing evidence of an epidemic? Hepatol Res. 2012;42(1):1-14.
- Bujold A, Dawson LA. Stereotactic radiation therapy and selective internal radiation therapy for hepatocellular carcinoma. Cancer Radiother. 2011;15(1):54-63.
- Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218(2):145-151.
- Orlando A, Cottone M, Virdone R, et al. Treatment of small hepatocellular carcinoma associated with cirrhosis by percutaneous ethanol injection. A trial with a comparison group. Scand J Gastroenterol. 1997;32(6):598-603.
- Matsui O, Kadoya M, Yoshikawa J, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188(1):79-83.
- Horigome H, Nomura T, Nakao H, et al. Percutaneous radio-frequency ablation therapy using a clustered electrode for malignant liver tumors. Clin Gastroenterol. 2001;32(5):418-422.
- Seong J. Challenge and hope in radiotherapy of hepatocellular carcinoma. Yonsei Med J. 2009 Oct 31;50(5):601-612.
- Pugh RN, Murray-Lyon IM, Dawson JL. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646-649.
- Klein J, Dawson LA, et al. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys. 2013;87(1):22-32.
- Lee IJ, Seong J. The optimal selection of radiotherapy treatment for hepatocellular carcinoma. Gut Liver. 2012;6(2):139-48. doi: 10.5009/gnl.2012.6.2.139.
- Pan CC, Kavanagh BD, Dawson LA. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S94-100.
- RTOG1112: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1112, May 2013.
- Soliman H, Ringash J, Jiang H. Phase II Trial of Palliative Radiotherapy for Hepatocellular Carcinoma and Liver Metastases. J Clin Oncol. 2013;31(31):3980-6
- Blomgren H, Lax I, Näslund I, Svanström R, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34(6):861-870.
- Guha C, Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol. 2011;21(4):256-263.
- Son SH, Choi BO, Ryu MR, et al. Stereotactic Body Radiotherapy for Patients with Unresectable Primary Hepatocellular Carcinoma: Dose-Volumetric Parameters Predicting the Hepatic Complication. Int J Radiat Oncol Biol Phys. 2010;78(4):1073-1080.
- Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26(4):657-664.
- Cárdenes HR, Price TR, Perkins SM, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12(3):218-225.
- Seo YS, Kim MS, Yoo SY, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol. 2010;102(3):209-214.
- Kwon JH, Bae SH, Kim JY, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. BMC Cancer. 2010;10(1):475.
- Louis C, Dewas S, Mirabel X, et al. Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treat. 2010;9(5):479-487.
- Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic Body Radiotherapy for Primary Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys. 2011;81(4):e447-453.
- Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: A retrospective outcome analysis in 185 patients. Acta Oncol. 2013 Aug. [Epub ahead of print]
- Jang WI, Kim MS, Bae SH, et al. High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol. 2013;8(1):250. [Epub ahead of print]
- Jung J, Yoon SM, Kim SY, et al. Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: clinical and dose-volumetric parameters. Radiat Oncol. 2013;8(1):249. [Epub ahead of print]
- Huang WY, Kao CH, Huang WS, et al. 18F-FDG PET and combined 18F-FDG-contrast CT parameters as predictors of tumor control for hepatocellular carcinoma after stereotactic ablative radiotherapy. J Nucl Med. 2013;54(10):1710-1716.
- Shin YJ, Kim MS, Yoo SY, et al. Pilot study of stereotactic body radiotherapy for huge hepatocellular carcinoma unsuitable for other therapies. Tumori. 2010;96(1):65-70.
- Goyal K, Einstein D, Yao M, et al. Cyberknife stereotactic body radiation therapy for nonresectable tumors of the liver: preliminary results. HPB Surg. 2010;2010. pii: 309780. doi: 10.1155/2010/309780. Epub 2010 Jun 28.
- Facciuto ME, Singh MK, Rochon C, et al. Stereotactic body radiation therapy in hepatocellular carcinoma and cirrhosis: Evaluation of radiological and pathological response. J Surg Oncol. 2012;105(7):692-698.
- Sandroussi C, Dawson LA, Lee M, et al. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int. 2010;23(3):299-306.
- Al Hamad AA, Hassanain M, Michel RP, et al. Stereotactic radiotherapy of the liver: a bridge to transplantation. Technol Cancer Res Treat. 2009;8(6):401-405.
- O’Connor JK, Trotter J, Davis GL, et al. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl. 2012;18(8):949-954.
- Bujold A, Massey CA, Kim J, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31(13):1631-1639.
- Mancosu P, Castiglioni S, Reggiori G, et al. Stereotactic body radiation therapy for liver tumours using flattening filter free beam: dosimetric and technical considerations. Radiat Oncol. 2012;7:16.
