A Case of Vision Loss From Radiation-Induced Optic Neuropathy Resulting in Charles Bonnet Syndrome
Images
Abstract
Radiation-induced optic neuropathy (RION) is a rare late effect following radiation caused by damage to the optic nerves or chiasm. It is a profound and devastating complication of radiation therapy with no effective treatment and is irreversible. Charles Bonnet syndrome (CBS) is a rare phenomenon characterized by complex visual hallucinations that occur concurrently with visual field loss or visual acuity loss. This case describes a woman with a CNS WHO grade 2 meningioma who received conventionally fractionated radiation therapy with a proton beam to the residual tumor and resection cavity after near total resection. She subsequently developed RION with vision loss and hallucinations and was diagnosed with CBS. We recommend that even though the incidence of RION is rare, patients should be counseled by providers for potential late effects of radiation treatment with surveillance routinely after treatment.
Keywords: Radiation toxicity, radiation-induced optic neuropathy, Charles Bonnet syndrome, proton beam radiation therapy
Case Summary
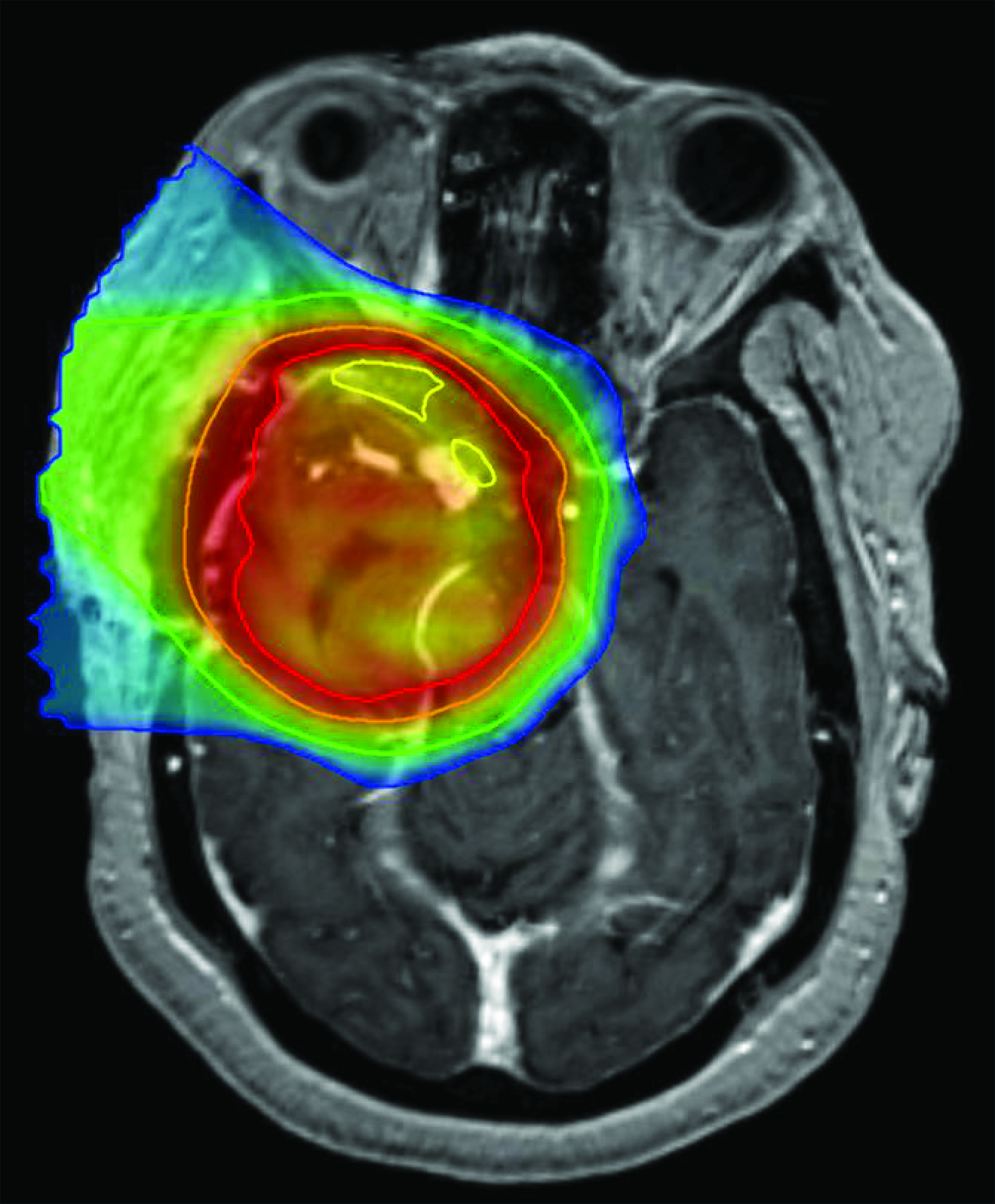
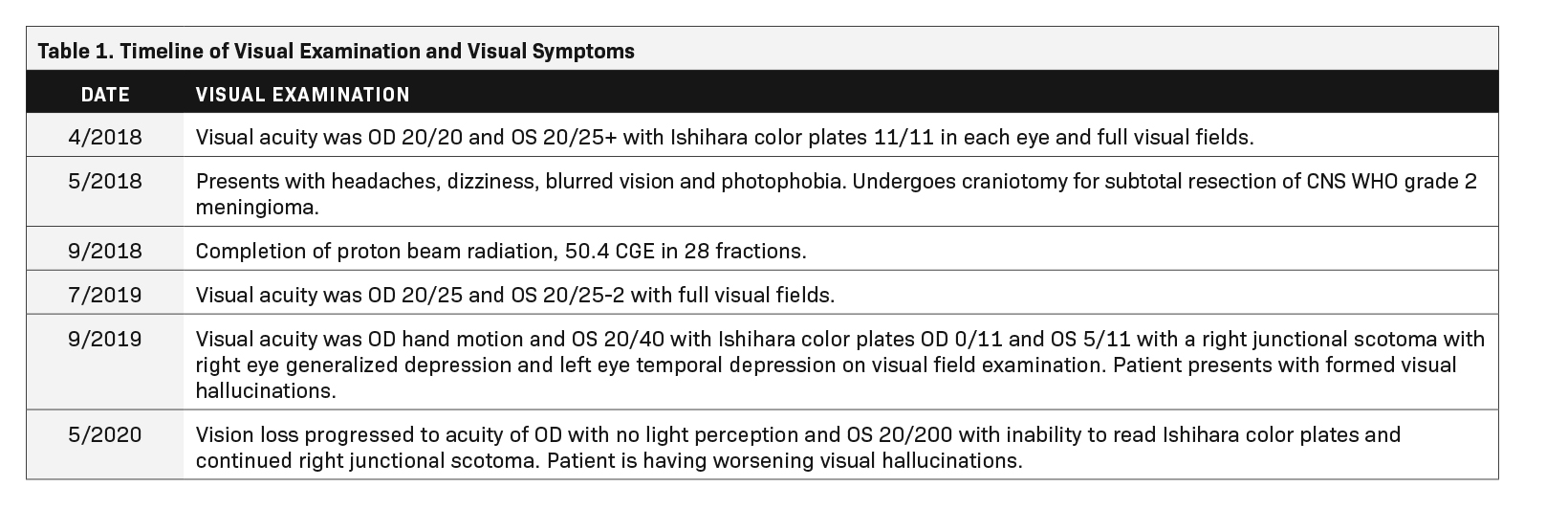
A 60-year-old Black woman with a history of hypertension, long-term use of hydroxychloroquine sulfate use for rheumatoid arthritis, migraine headaches, and bilateral cataract extractions, presented to the emergency department with dizziness, headache, and vision changes consisting of photophobia and blurred vision. Of note, the patient had been seen for a routine ophthalmic examination the month prior with noted dry eyes, stable pseudophakia OU (oculus uterque or each eye) and myopia with astigmatism and presbyopia. She also had been on long-term use of hydroxychloroquine for 18+ years with no changes on examination. Visual acuity was OD 20/20 and OS 20/25+ with Ishihara color plates 11/11 in each eye and full visual fields. (See Table 1 for a timeline of visual examinations and symptoms.) Brain MRI with and without contrast demonstrated a 3.5-cm extra-axial mass in the base of the skull arising in the right middle cranial fossa located medially in the para-cavernous region. The patient underwent a near total resection of the tumor and pathology showed a CNS WHO grade 2 meningioma. One month following surgery, she completed conventionally fractionated radiation therapy to the residual tumor and surrounding high-risk region using proton beam therapy to a total dose of 50.4 cobalt gray equivalents (CGE) in 28 fractions (1.8 CGE per fraction) (Figure 1). In the absence of an established dose response and given the proximity of the optic apparatus and the emerging uncertainty about proton relative biological effectiveness (RBE) and radiation injury, a dose of 50.4 CGE was used. The maximum (to 0.03 cc volume) and mean doses to the optic nerves and chiasm were: right optic nerve max 51.86 CGE/mean 26.35 CGE, left optic nerve max 39.69 CGE/mean 6.09 CGE, and optic chiasm max 52.09 CGE/mean 50.85 CGE. Following radiation treatment the patient reported persistent right-sided facial numbness and tingling with intermittent headaches.
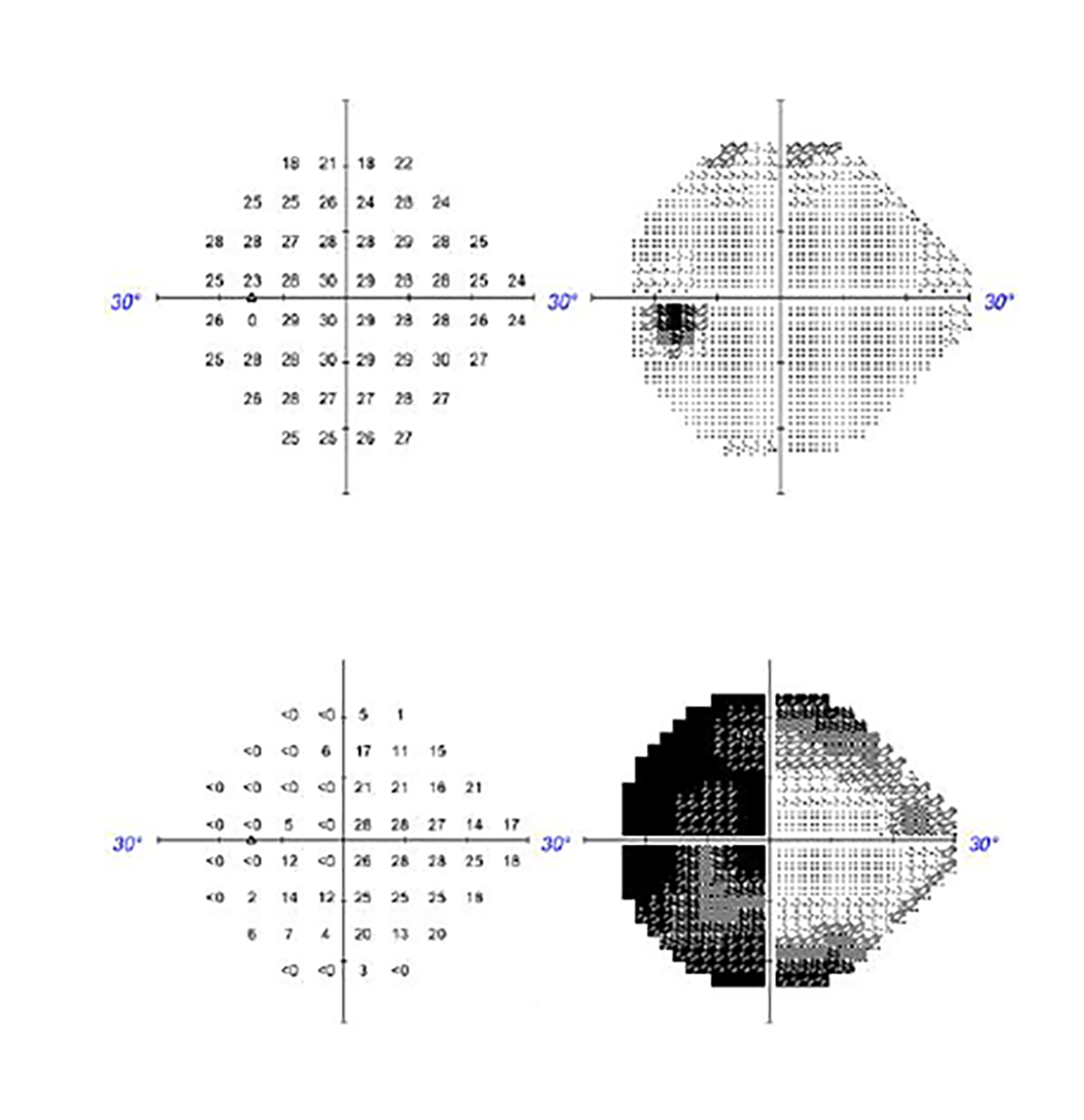
Approximately 1 year after treatment, the patient complained of a pressure sensation inside her head and further decrease in vision, worse in the right eye. Two months prior to presenting with these symptoms, visual acuity was OD 20/25 and OS 20/25-2 with full visual fields. The first examination after onset of radiation-induced optic neuropathy (RION) showed visual acuity OD hand mo- tion and OS 20/40 with Ishihara color plates OD 0/11 and OS 5/11 with a right junctional scotoma with right eye generalized depression and left eye temporal depression on visual field examination. She also reported having hallucinations consisting of geometric shapes, lions with manes, and women’s faces occurring primarily when her eyes were closed or before sleep at night, and upon awakening. MRI did not show obvious evidence of recurrent meningioma but showed areas of nonspecific enhancement in the bilateral intracranial optic nerves greater on the right, concerning for radiation optic neuropathy. She was started on dexamethasone, vitamin E, pentoxifylline, and bevacizumab. She was also started on nortriptyline, which helped with the pain on right side of her head. Bevacizumab was discontinued after 2 doses due to perforated diverticula. After a month of treatment, she reported mild improvement in the left eye but continued to have poor vision in the right eye. An ophthalmology examination showed improvement in acuity and foveal threshold with stable to mildly worse results on visual field testing (Figure 2). Vision loss progressed to acuity of OD with no light perception and OS 20/200 with inability to read Ishihara color plates and continued right junctional scotoma. The patient continued to have worsening visual hallucinations.
Diagnosis
In this patient’s case, findings were consistent with RION. She was evaluated by a neuro-ophthalmologist and diagnosed with Charles Bonnet syndrome as a result of RION. Two years after radiation, her vision continued to deteriorate with almost no light perception in the right eye and greatly diminished vision in left eye although she was able to make out light and shapes, sometimes seeing red flashes from the right eye. Hallucinations increased over time as vision deteriorated. She declined medical treatment due to possible side effects and possible interactions with medications for her comorbidities.
Imaging Findings
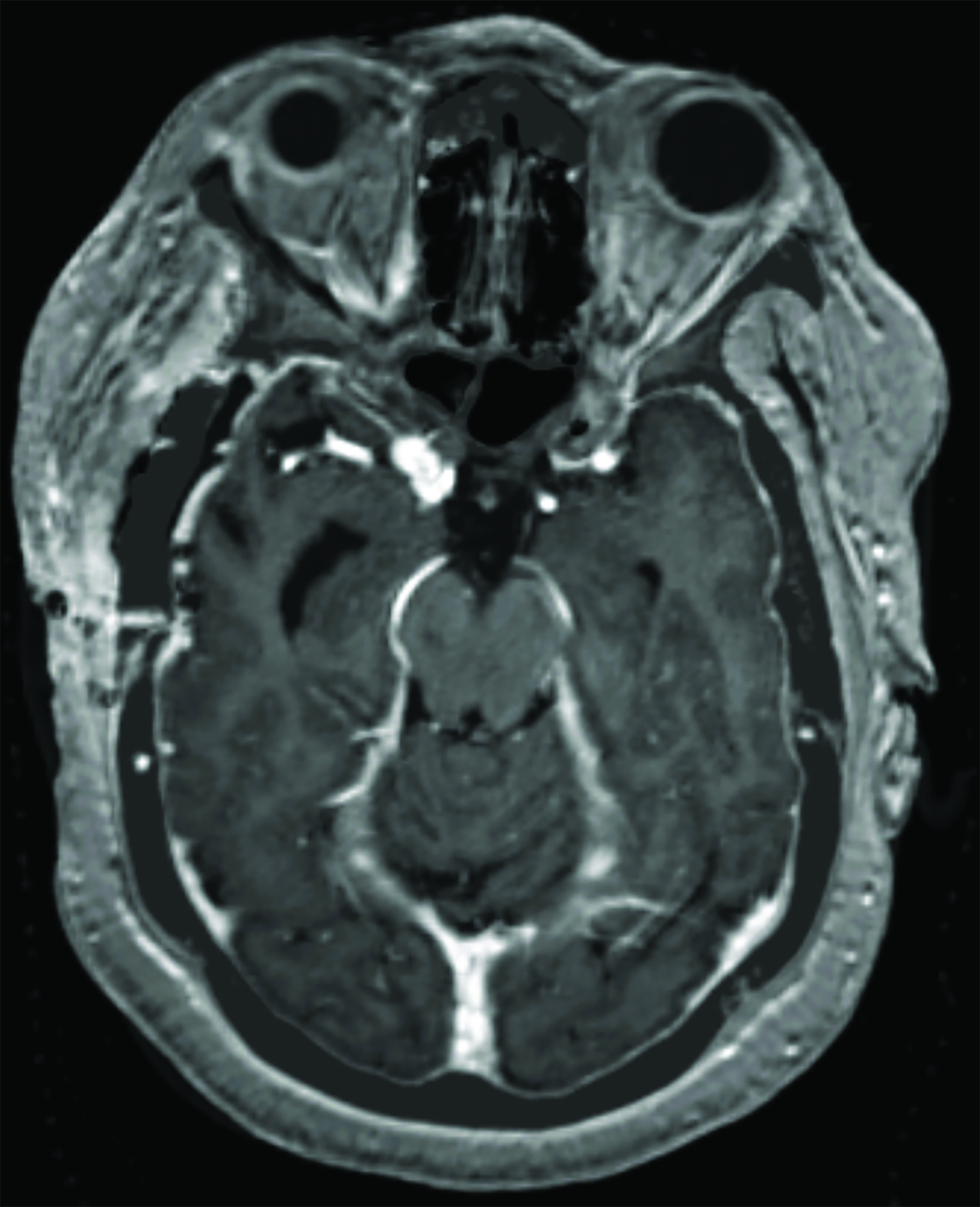
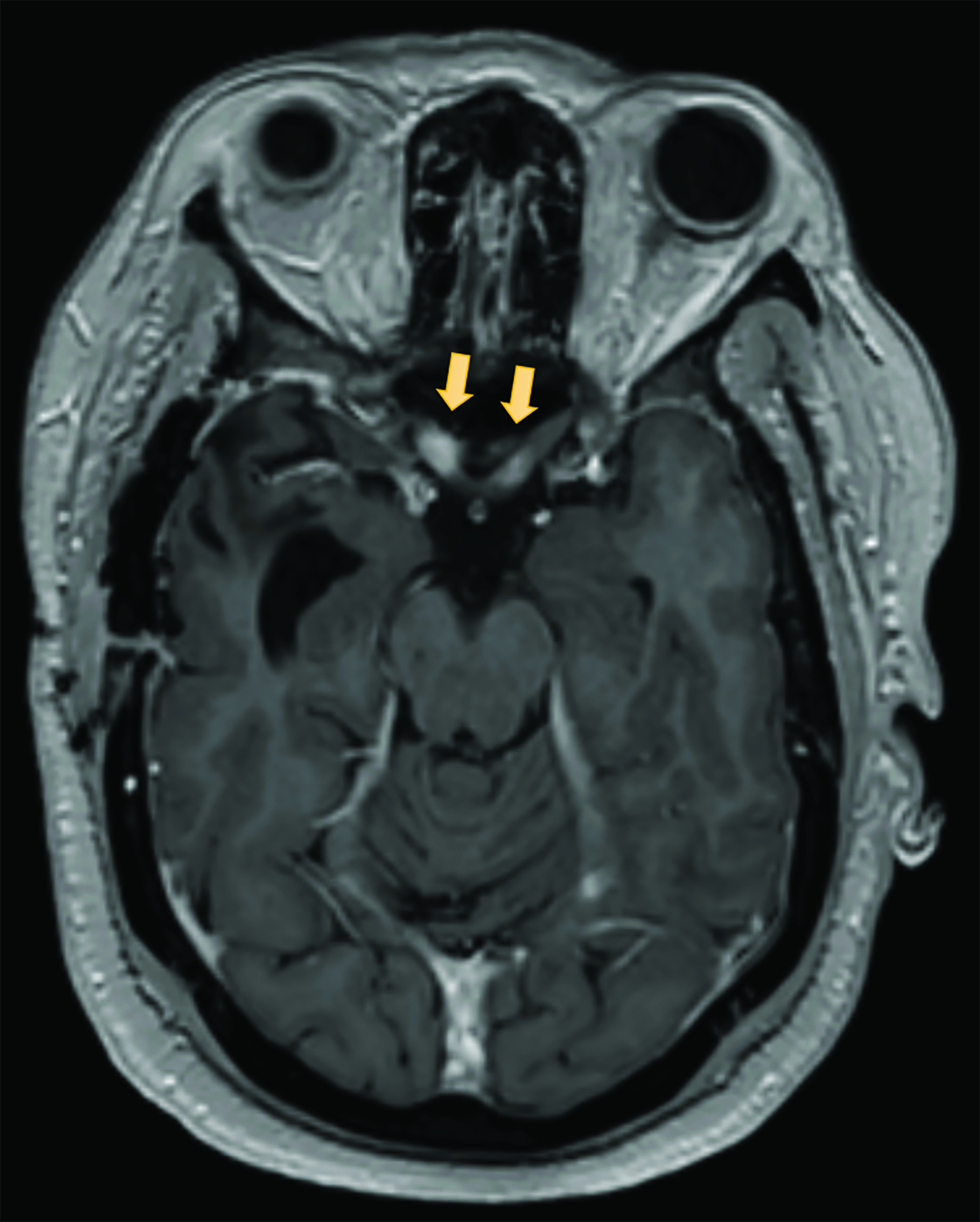
A preoperative MRI of the brain revealed encroachment of the meningioma upon the region of the right cavernous sinus. The right carotid terminus and right M1 segment appeared to be anteriorly displaced by the mass. There was a moderate amount of hyperintense FLAIR (fluid attenuated inversion recovery) signal within the surrounding brain parenchyma suggestive of edema. There was mild encroachment upon the suprasellar cistern with mass effect on the right aspect of the optic chiasm and right mammillary body. A postoperative MRI demonstrated dural thickening deep to the craniotomy bed and over the anterior right hemisphere most prominently over the right temporal lobe (Figure 3). An MRI 14 months after radiation with high-resolution images through the orbits demonstrated abnormal increased signal on the STIR (short tau inversion recovery) images with corresponding abnormal enhancement on the postgadolinium images involving the intracranial and intracanalicular segment of the right optic nerve and intracranial segment of the left optic nerve (Figure 4).
Discussion
CBS is caused by damage to the optic pathway resulting in visual hallucinations. It has not been well described in the setting of damage from radiation therapy. CBS was first described in 1760 by Swiss scientist Charles Bonnet when his 90-year-old grandfather experienced hallucinations after his vision deteriorated following cataract surgery.1 There have been a number of definitions of CBS since the original description; however, the most widely accepted definition is the Gold and Rabins’ definition, which describes hallucinations as stereotyped, formed, varied in complexity, persistent, or repetitive in nature.2,3 The deafferentation theory, or sensory deprivation theory, is the most widely accepted theory elucidating the phenomenon associated with CBS whereby loss of sensory vi- sual input is accompanied by increased excitability within the visual association cortex resulting in visual hallucinations.1,4-6 Visual hallucinations may be a consequence of ocular or optic pathway pathology and subsequent deterioration in vision. The incidence of CBS is variable and ranges from 0.4% to 30% with statistically higher incidence noted with worsening visual acuity.1 In a study of 100 consecutive patients with macular choroidal neovascularization, Brown et al noted increased incidence of formed hallucinations in patients with macular degeneration associated with bilateral choroidal neovascularization.4 Moreover, patients with a more sudden onset of vision loss are more likely to experience hallucinations than those experiencing a more gradual loss of vision.4,7
Visual hallucinations of CBS may include people, animals, buildings, landscapes, and geometric designs.1,7 Hallucinations may last for a few seconds to hours and may be simple or more complex in nature. Patients are typically aware that they are fictitious in nature and do not pose a threat. Patients may also report feeling stressed with hallucinations; however, this may stem more from worry regarding possible causes of the hallucinations vs the actual hallucination. Of note, patients with CBS experience some level of impaired vision and often report that hallucinations have better clarity than any residual vision.1,4 There is no age limit in CBS, although it most often affects older people due to an increase in eye diseases. Hallucinations in children are similar to adults and have been reported as images such as flashing lights, people’s faces, houses, animals, ballerinas, snowballs and colored balls.8 Visual hallucinations associated with CBS may eventually disappear spontaneously and also with complete blindness; however, they may persist for years in some cases.1,7 In a large prospective study conducted in the Netherlands, Teunisse et al found that hallucinations disappeared as blindness progressed in patients with macular and corneal degeneration.9 Frequency and duration of episodes may decrease over time and some studies have suggested people may get used to hallucinations over time.
Radiation-induced optic neuropathy is a rare late effect following radiation, caused by damage to the optic nerves or chiasm and is thought to result from radiation-induced microangiopathy associated with endothelial cell loss resulting in demyelination.10,11 Symptoms may be seen several months to several years after treatment and may lead to unilateral or bilateral blindness.10 RION typically presents in the clinical setting with onset of visual symptoms in the majority of patients within 3 years of therapy completion.12 Peak incidence of RION is 1 to 1.5 years after completion of radiation and is associated with characteristic findings on MRI with gadolinium contrast, which demonstrates marked enhancement of the optic nerve and chiasm on T1-weighted images.10,12 Both eyes are often involved serially. Consequently, vision in both eyes should be evaluated at the earliest onset of vision loss.12 There is no established effective treatment for RION.12,14 A few small studies have suggested the use of hyperbaric oxygen treatment if initiated within 72 hours of visual loss, but results have been limited.12,14 Treatment with steroids, pentoxifylline with vitamin E, and bevacizumab have also had limited benefit.10,12,14
Radiation tolerance to optic structures is a critical component and cumulative doses that exceed a fractionated schedule of 55 Gy up to 60 Gy, with fractions of 1.9 Gy or less, or a single dose of 10-12 Gy, are associated with increased risk factors for developing RION.10,13,14 Proton doses are defined in terms of Gy, or cobalt Gray-equivalent (CGE), with relative biologic effectiveness equaling proton Gy x 1.1.13 The risk of developing RION above these thresholds is approximately 5%.13,15 Some patients have reported developing vision loss at lower doses.10,19 RION occurs with different radiation modalities; however, proton therapy has been of particular interest in the treatment of tumors involving these structures due to the ability to deliver higher doses while sparing organs at risk (OARs) near the treatment field. Other contributing risk factors in the development of RION include older age, female sex, optic nerve compression, chemotherapy, previous radiation, hypertension, diabetes mellitus, hyperlipidemia, and smoking history.11-13,16 Additionally, pre-existing compression of the optic nerves and chiasm may predispose these structures to RION.12 The diagnosis of CBS is one of exclusion and other etiologies should be investigated to determine possible treatment modalities. Patients who have received radiation and have signs and symptoms of RION should be referred to ophthalmology emergently as well as to a neuro-oncology team for initial assessment and management if diagnosis is confirmed. Antipsychotics, anticonvulsants, anti-anxiety medication, and selective serotonin reuptake inhibitors have been reported as treatments for CBS. However, none of these have been shown to be particularly effective for CBS.1,17,18 Behavioral techniques such as blinking during hallucination or rapid eye movement from one object to another, away from the hallucination field of vision, may be helpful in suppressing hallucinations.7
Even though the incidence of RION is rare, patients should be counseled by providers for potential late effects of radiation treatment with surveillance routinely following treatment. Older patients with ocular diseases and other comorbid risk factors should be closely monitored. Ophthalmologists and other optical providers should be aware of the potential for visual hallucinations in patients with visual impairment and optical pathology. Early recognition of CBS symptoms can lessen distress and anxiety experienced by patients with CBS.
References
- Pang L. Hallucinations experienced by visually impaired: Charles Bonnet syndrome. Optom Vis Sci. 2016;93:1466-1478. doi:10.1097/OPX.0000000000000959
- Ffytche, DH. Visual hallucinatory syndromes: past, present, and future. Dialogues Clin Neurosci. 2007;158:1158-1159. doi:10.31887/DCNS.2007.9.2/dffytche
- Boyer P, Devlin M, Boggild M. Rare and rarer: co-occurrence of stroke-like migraine attacks after radiation therapy and Charles Bonnet syndromes. Oxf Med Case Reports. 2018;10:349-352. doi:10.1093/omcr/omy077
- Brown GC, Murphy RP. Visual symptoms associated with choroidal neovascularization. Arch Ophthalmol. 1992;1101251-1101256. doi:10.1001/ar- chopht.1992.01080210069027
- Firbank MJ, DaSilva Morgan K, Cikkertibm D, et al. Investigation of structural brain changes in Charles Bonnet syndrome. Neuroimage Clin. 2022;35:103041 doi:10.1016/j.nicl.2022.103041
- DeSouza RK, Martins RT, Kowacs, PA. Charles Bonnet syndrome: complete remission of visual hallucinations with Trazodone. Neuropsychiatry. 2019;9(2):2305-2307. doi:10.4172/NEUROPSYCHIATRY.1000578
- Kester EM. Charles Bonnet syndrome: Case presentation and literature review. Optometry. 2009;80:360-366. doi:10.1016/j.optm.2008.10.017
- Schwartz TL, Vahgei L. Charles Bonnet syndrome in children. J AAPOS. 1998;2(5):310-313. doi:10.1016/s1091-8531(98)90091-x
- Teunisse RJ, Cruysbert JR, Verbeek A, Zitman FG. The Charles Bonnet syndrome: a large prospective study in the Netherlands. Br J Psych. 1995;166:254-257.
- Freiman TM, Surges R, Vougioukas VI, et al. Complex visual hallucinations (Charles Bonnet syndrome) in visual field defects following cerebral surgery. JNeurosurg. 2004;101:846-853. doi:10.3171/jns.2004.101.5.0846
- Doroslovack P, Tamhankar MA, Liu GT, Shindler KS, Ying G, Alonso-Basanta M. Factors associated with occurrence of radiation-induced optic neuropathy at “safe” radiation dosage. Sem Ophthalmol. 2018;33(4):581-588. doi:10.1080/08820538.2017.1346133
- Danesh-Meyer, HV. Radiation-induced optic neuropathy. J Clin Neurosci. 2008;15(2)95-100. doi:10.1016/j.jocn.2007.09.004
- Kothe A, Feuvret L, Weber DC, Safai S, Lomax AJ. Assessment of radiation-induced optic neuropathy in a multi-institutional cohort of chordoma and chon- drosarcoma patients treated with proton therapy. Cancers. 2021;13:5237 doi:10.3390/cancers13215327
- Indaram M, Ali FS, Levin MH. In search of a treatment for radiation-induced optic neuropathy. Curr Treat Options Neurol. 2015;17(1):325. doi.org/10.1007/ s11940-014-0325-2
- Li PC, Liebsch NJ, Niemierko A, et al. Radiation tolerance of the optic pathway in patients treated with proton and photon radiotherapy. Radiother Oncol. 2019;131:112-119. doi:10.1016/j.radonc.2018.12.007
- Kountouri M, Pica A, Walser M, et al. Radiation-induced optic neuropathy after pencil beam scanning proton therapy for skull-base and head and neck tumours. Br J Radiol. 2020;93:20190028. doi:10.1259/bjr.20190028
- Hori H, Terao T, Shiraishi Y, Nakamura J. Treatment of Charles Bonnet syndrome with valproate. Int Clin Psychopharmacol. 2000;15(2)117-119. doi:10.1097/00004850-200015020-00009.
- Lang UE, Stogowski D, Schulze D, et al. Charles Bonnet syndrome: successful treatment of visual hallucinations due to vision loss with selective serotonin reuptake inhibitors. J Psychopharm. 2007;21(5)477-485. doi:10.1177/0269881106075275
- Siddiqui JD, Loeffler JS, Murphy, MA. Radiation optic neuropathy after proton beam therapy for optic nerve sheath meningioma. J Neuro-Ophthalmol. 33(2):165-168. doi:10.1097/WNO.0b013e31828292b8
Citation
Sandstrom SK, Mansur DB, Choi, MLMS. A Case of Vision Loss From Radiation-Induced Optic Neuropathy Resulting in Charles Bonnet Syndrome. Appl Radiat Oncol. 2023;(2):34-38.
July 4, 2023