A Novel Framework to Define and Prognosticate Visual Outcomes Following Fractionated Radiation Therapy for Optic Nerve Sheath Me
Introduction
Optic nerve sheath meningiomas (ONSMs) are rare tumors located within the orbital canal, accounting for only 1% to 2% of all meningiomas. Despite their rarity, they are the second most common primary tumor found in the orbit, representing a third of all optic nerve tumors.1 Typically, ONSMs cause a painless and gradual loss of vision, most commonly in middle-aged women. Although a classic triad of vision loss, optic nerve atrophy, and an optociliary shunt is often used to describe ONSM, in practice one or more of these features may be absent at presentation.2,3 Radiographically, ONSMs can be differentiated from more common gliomas by the presence of calcifications and a “tram-track sign” on fat-suppressed T1 gadolinium-enhanced scans.4 Due to their characteristic appearance on imaging and well-established clinical presentation, biopsies are generally not necessary for diagnosis or treatment and may even be harmful.5,6 Intervention may indeed be a cat-and-mouse game with some meningiomas, but when it comes to the management of ONSMs, the most important consideration is unequivocally the preservation of vision.
With regard to the watch-and-wait strategy used in the management of OSNMs, the decision tree for when to proceed with intervention can be tricky. ONSMs are insidious as they can progress slowly and without symptoms. However, if left untreated, they can eventually lead to complete blindness in the affected eye due to compression of the optic nerve.7 In some cases, patients may retain good vision and have minimal visual field (VF) loss even without prolonged nerve compression. Therefore, serial ophthalmological examinations are necessary to monitor for visual loss and optic nerve atrophy, and most ONSMs can be managed conservatively with a watch-and-wait strategy for a number of years.2
Intervention in the form of RT and/or surgery is typically reserved for cases of high-risk tumor progression or deterioration of eyesight.8 Surgery is usually only considered for cases of morbid proptosis in patients who have experienced severe visual loss as it carries a high risk of compromising the blood supply to the optic nerve and causing iatrogenic visual loss.1,9,10 Currently, fractionated stereotactic RT (FSRT) is considered the standard of care for ONSMs.
Many studies evaluating FSRT for ONSMs have a relatively short follow-up, and few report outcomes using complete ophthalmological examinations.11 Therefore, we strongly believe that a comprehensive assessment of visual acuity (VA), VF, and color vision (CV) is necessary to obtain a pertinent picture of the patient’s visual status leading up to treatment and in the years following treatment. Currently, no guidelines use comprehensive visual examination outcomes to define a composite visual outcome after definitive treatment of ONSM. Hence, we propose a new standard to categorize visual outcomes after FSRT as improved, worsened, or unchanged. In our cohort, we evaluate factors such as patient age, time to intervention, VF, CV, VA, and radiation specifications to assess visual outcomes. We hope that our framework will contribute to decision-making and serve as a foundation for further meta-analyses and larger cohorts to redefine their visual outcomes and predict outcomes for their patients. The results will provide physicians and patients with information to guide decision-making and manage expectations for visual outcomes following treatment with FSRT.
Materials and Methods
Patient Selection
This retrospective study underwent approval by the institutional review boards of both Thomas Jefferson University and Wills Eye Hospital. The study focused on reviewing radiation records from patients treated for ONSMs between 1997 and 2012, ensuring long-term follow-up. To be considered for analysis, patients had to have been treated with FSRT, with comprehensive follow-up, including both MRI and ophthalmological assessments before and after treatment. Patients who initially presented with blindness or whose meningiomas showed evidence of extra-orbital origin were excluded. A total of 29 primary ONSM cases from 27 patients met the inclusion criteria. One patient received a second course of RT 3.5 years after initial treatment for tumor control, and another patient had bilateral ONSMs (clinically suspected neurofibromatosis) treated simultaneously. An overall summary of each patient is provided in
Data Recording
To gather relevant information for the study, patient charts were reviewed in paper and electronic formats. The information collected included demographic, medical, radiographic, and ophthalmologic data such as VF, VA, CV, and proptosis. Radiation therapy information such as dose, fractionation schedule, maximum dose to structure and optic nerve, and maximum dose to prescription dose ratio (MDPD) was also recorded. Visual acuity was measured using Snellen notation, color recognition was documented by the number of Ishihara plates identified, and visual defects were determined by the ophthalmologist’s interpretation of automated VFs using the Humphrey Visual Fields analyzer and Matrix 24-2 program from Zeiss.
Radiation Treatment Details
Prior to 2004, the X-Knife 3-D planning system (Radionics) was utilized for treatment planning, and a dedicated stereotactic 600SR linear accelerator (Varian) was employed for radiation treatment delivery. Between 2004 and 2013, iPlan (Brainlab) was used for treatment planning, and TrueBeam STx (Varian) equipped with high-definition multileaf collimators and ExacTrac (Brainlab) for onboard imaging was used for treatment delivery. All patients receiving irradiation were immobilized using custom-made Brainlab thermoplastic masks.
For all patients, treatment planning MRI (thin cut [1-1.5 mm] axial fat-suppressed postcontrast MRI) and CT images were obtained and fused. The gadolinium-enhanced T1-weighted series was used to define the gross tumor volume (GTV) on MRI. The planning target volume (PTV) was defined as the GTV with a minimum margin of 0-2 mm, as determined by the treating physician. Critical normal structures, such as the optic nerves, chiasm, and brainstem, were contoured as organs at risk.
Intensity-modulated radiation therapy (IMRT) or hybrid arcs (a combination of dynamic arcs with static IMRT beams) were employed as the radiation planning modality prior to 2004.
Defining Visual Status and Outcomes
According to the World Health Organization’s (WHO’s) “Levels of Visual Impairment” guidelines, visual impairment (based on VA only) is categorized as follows using Snellen notation: 20/20 to 20/30 = minimal to no impairment, 20/40 to 20/70 = mild, 20/80 to 20/160 = moderate, 20/200 to light perception = severe, and no light perception = blindness.12 Visual field defects were categorized as small if they encompassed < 50% of the VF or large if > 50%. Color vision was classified as deficient or full, depending on the presence or absence of a deficit in any of the Ishihara color plates. The VA, VF, and CV of the uninvolved eyes were also evaluated.
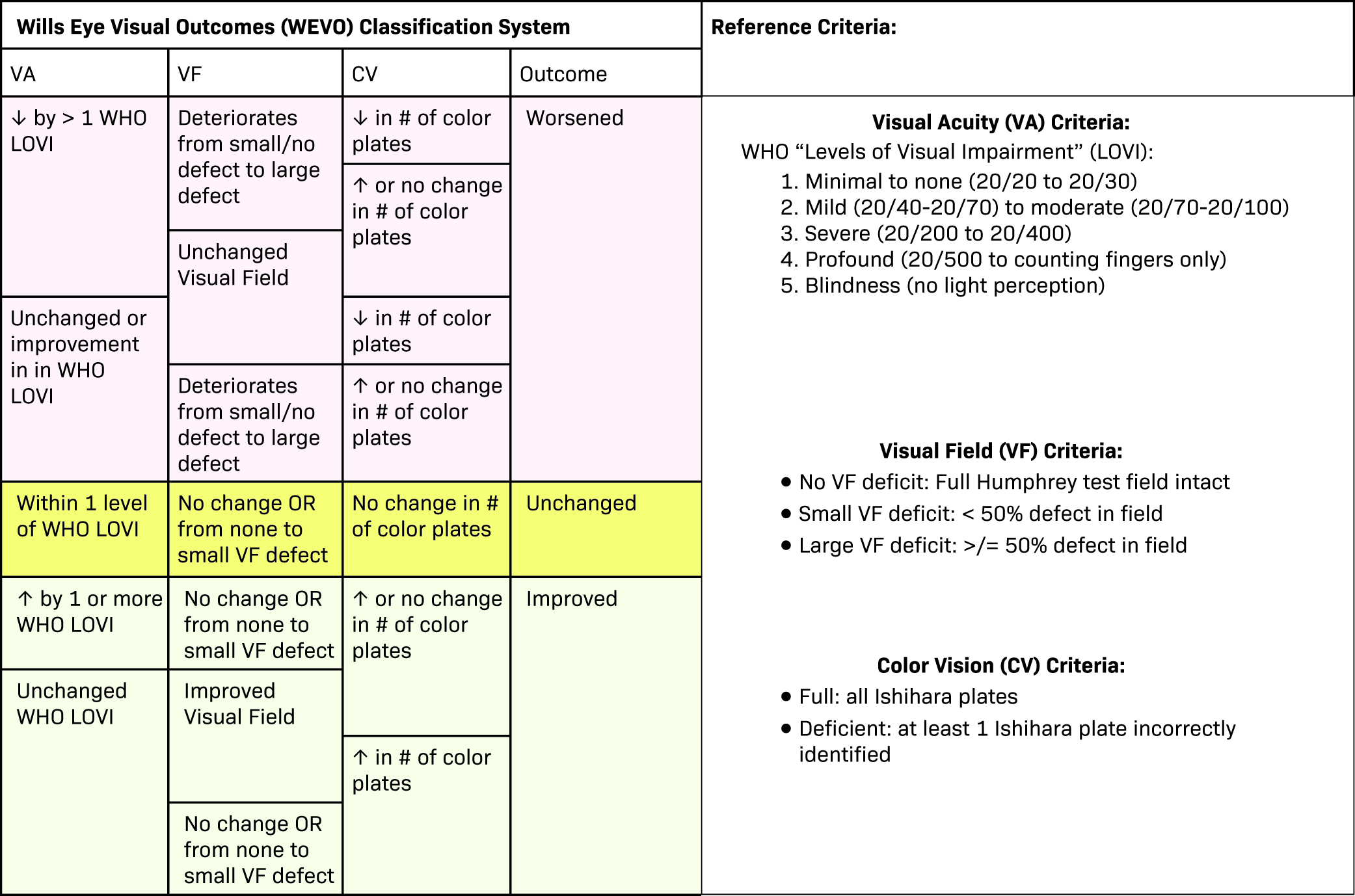
As no established criteria are available in the literature to combine VA, VF, and CV into a comprehensive endpoint, we propose a new system to classify “visual outcome” as either worsened, unchanged, or improved. The Wills Eye Visual Outcomes (WEVO) classification system (shown in
Worsened vision if 1 or more of the following are met:
VA deteriorates by >1 WHO “Level of Visual Impairment.”
VF deteriorates from small to large defects.
Deficient in color plate interpretations.
Unchanged vision if
VA remains within 1 WHO level of visual impairment.
No change in VF, or only develops a small defect.
No change in the number of color plate interpretations.
Improved vision if any of the following are met:
VA improvement by 1 or more WHO levels of visual impairment with no change in VF or CV.
Improvement of VA with improvement of VF and CV.
Stable VA within 1 WHO level, but with improvement of either or both VF and CV.
Wills Eye Visual Outcomes (WEVO) classification system. We introduce the WEVO classification system, designed to evaluate the impact of therapy on optic nerve tumors, specifically focusing on visual acuity (VA), visual field (VF), and color vision (CV). The reference criteria for VA, VF, and CV are provided on the right side of the figure. To assess visual outcomes, users can systematically navigate through each column, following the categories from left to right. This framework allows for a comprehensive evaluation of whether visual outcomes have improved, remained unchanged, or worsened after therapy.
Within our cohort, each case was subsequently classified as worsened, improved, or unchanged as seen in
Summary of Visual Outcomes
| CASE | OVERALL STATUS | RATIONALE | CASE | OVERALL STATUS | RATIONALE |
|---|---|---|---|---|---|
| 1 | = | Unchanged VA; unchanged VF; unchanged CV | 14 | + | Improved VA; unchanged VF; unchanged CV |
| 2a | + | Unchanged VA; improved VF; unchanged CV | 15 | + | Improved VA; unchanged VF; unchanged CV |
| 2b | = | Unchanged VA; unchanged VF; unchanged CV | 16 | − | Worsened VA; mildly improved VF defect; unchanged CV |
| 3 | = | Insignificant change in VA; unchanged VF; unchanged CV | 17 | − | Worsened VA; unchanged VF; unchanged CV |
| 4 | = | Unchanged VA; unchanged VF; unchanged CV | 18 | + | Improved VA; unchanged VF; unchanged CV |
| 5 | = | Insignificant change in VA; unchanged VF; unchanged CV | 19 | + | Improved VA; unchanged VF; improved CV |
| 6a | = | Unchanged VA; unchanged VF; unchanged CV | 20 | + | Improved VA; unchanged VF; unchanged CV |
| 6b | = | Unchanged VA; unchanged VF; unchanged CV | 21 | + | Improved VA; improved VF; improved CV |
| 7 | − | Worsened VA; worsened VF; unchanged CV | 22 | − | Worsened VA; unchanged VF; unchanged CV/unable to assess |
| 8 | + | Unchanged VA; improved VF; improved CV | 23 | + | Unchanged VA; improved VF; unchanged CV |
| 9 | = | Unchanged VA; unchanged VF; unchanged CV | 24 | − | Unchanged VA; worsened VF; unchanged CV/inability to assess |
| 10 | + | Improved VA; unchanged VF; unchanged CV | 25 | = | Unchanged VA; unchanged VF; unchanged CV/unable to assess |
| 11 | = | Insignificant change in VA; unchanged VF; unchanged CV | 26 | − | Worsened VA; unchanged VF; unchanged CV/unable to assess |
| 12 | + | Improved VA; unchanged VF; unchanged CV | 27 | − | Worsened VA; worsened VF; unchanged CV/unable to assess |
| 13 | − | Worsened VA; worsened VF; worsened CV |
Rationales for improved, unchanged, or worsened vision are given in detail. For more information on the levels of improvement and description of exam findings, see
Abbreviations: CV, color vision; VA, visual acuity; VF, visual field.
Statistics
Statistical analyses were conducted using GraphPad Prism version 5. Chi-squared tests were utilized to compare the outcomes between the worsened and improved/unchanged categories. For continuous variables such as maximum dose to the tumor or optic nerve, lesion size, and MDPD, a
Results
Patient Demographics and Presentation
Patient Demographics
| Patients ( | 27 |
| Male (%) | 4 (15%) |
| Female (%) | 23 (85%) |
| Laterality | |
| 9 (with 2 separate courses) | |
| 16 | |
| 1 (each side treated once) | |
| Age (y) at treatment: median (range) | 46 (33-73) |
| Total RT courses | 29 |
| Surgery then RT | 1 |
| Biopsy then RT | 1 |
| Single course of RT | 25 patients (25 cases) |
| Two separate courses of RT (same orbit) | 1 patient (2 cases) |
| Two separate courses of RT (bilateral) | 1 patient (2 cases) |
| Radiographic follow-up (mo) by MRI: median (range) | 77.2 (10-161) |
| Ophthalmological follow-up (mo) by MRI: median (range) | 81 (17-240) |
This information is a representative breakdown of the 27 patients and 29 cases of ONSM followed in this study, as well as the length of radiographic and visual follow-up.
Abbreviations: ONSM, optic nerve sheath meningioma; RT, radiation therapy.
Radiation Treatment
Most patients were treated with conformal dynamic arcs. The median total radiation dose was 52.2 Gy (range, 41.4-55.8) in 23-30 fractions, and the median maximum dose to the PTV was 61.8 Gy (range, 52.44-79.3). The median PTV size was 1.54 cc (range, 0.31-7.36 cc) and the MDPD was 1.18 (range, 1.04-1.48). While a high dose to the involved optic nerve was largely unavoidable given the nature of treating ONSMs, the optic chiasm dose was kept within an acceptable range with a goal of
Treatment and Overall Radiographic and Vision Outcomes
Radiation therapy was administered empirically in most cases, as ONSM was primarily diagnosed radiographically. Of the treated patients, 1 had a marginal recurrence and required re-treatment with radiation. The median duration of visual follow-up was 81 months (range, 17-240 mo). Of the 27 patients, 14 were initially observed prior to treatment, with a median observation period of 5 months (range, 0-180 mo) between diagnosis and treatment. The median MRI follow-up period was 77.2 months (range, 10-161 mo).
Radiographic control was achieved in 28/29 (96.6%) cases with 1 course of radiation and ultimately, all patients showed complete tumor control following RT, as assessed by MRI. There were no in-field ONSM recurrences. All patients were classified using WEVO criteria, and of the 29 optic nerves treated with FSRT, 11 (39%) showed visual improvement, 10 (34%) remained unchanged, and 8 (27%) experienced worsened vision.
Predictors of Visual Outcome
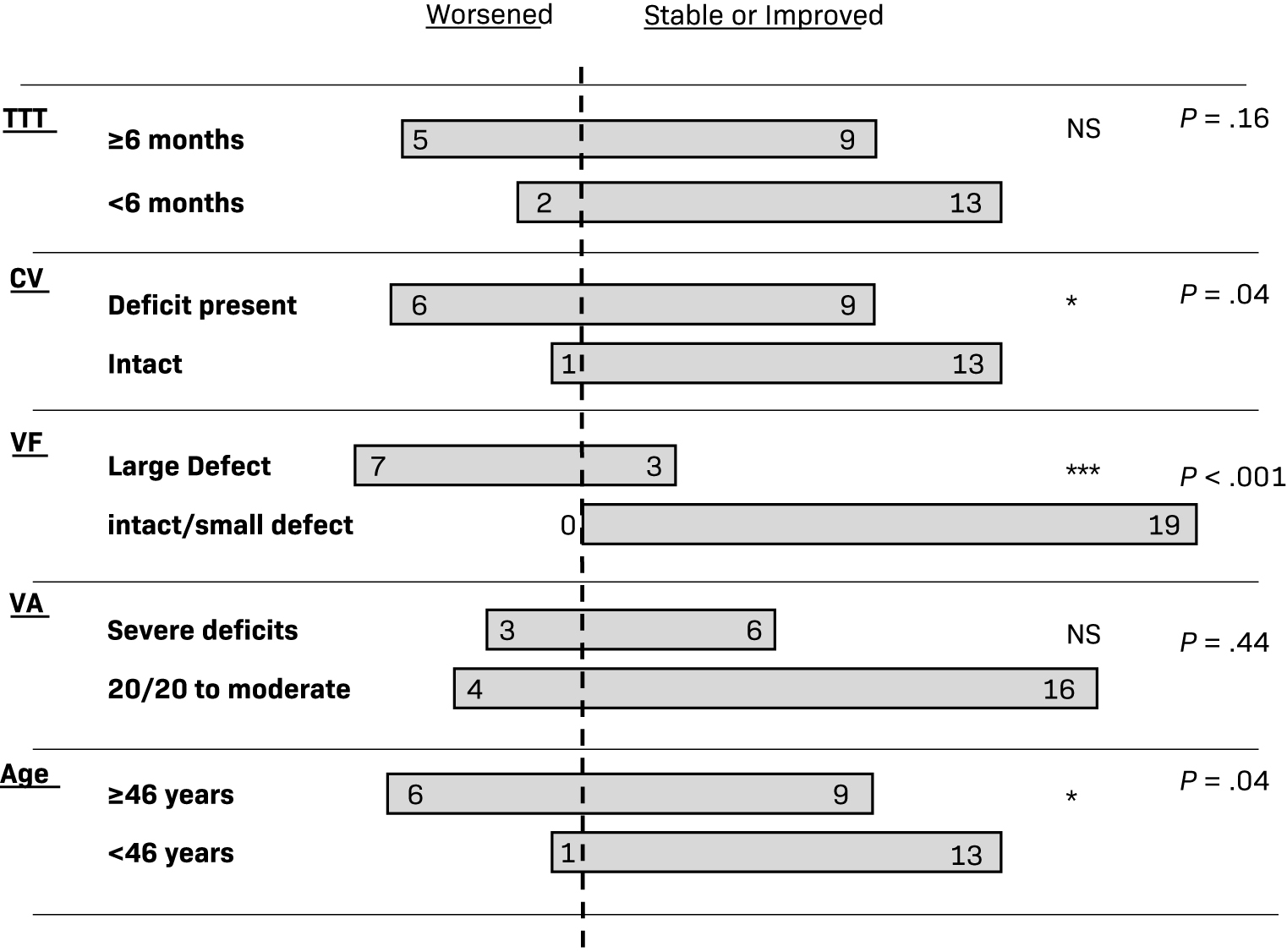
Time to treatment (TTT), CV, VFs, VA, and age at treatment were all assessed as potential predictors of visual outcomes following radiation treatment.
Assessment of time to treatment (TTT), color vision (CV), visual field (VF), visual acuity (VA), and age (in years) as pretreatment predictors of visual outcome. TTT was dichotomized into those who received radiation therapy (RT) ≥ 6 mo after diagnosis vs those who proceeded to treatment within 6 mo of diagnosis. CV was defined as deficient if any Ishihara plate was misread in the pretreatment ophthalmological exam. Intact denotes that all plates were read correctly. For VF, large defect denotes any field cut > 50%. A small defect was < 50%/VA was defined as severe deficits if vision was noted to be 20/200 or worse.
Time to Treatment
Those with a TTT of < 6 months from initial presentation showed worsened visual outcomes in 2 of 13 cases compared with 5 of 9 cases whose TTT was 6 months or greater (
Color Vision
For patients who had CV defects, 6 out of 15 had worsened visual outcomes compared with 1 out of 14 that showed worsening with full CV at presentation (
Visual Field
Of the 19 patients who presented with no defect or a small VF defect, none experienced worsened visual outcomes, while 7 of the 10 (70%) cases initially presenting with large visual defects experienced worsened visual outcomes (
Visual Acuity
Of the 20 patients whose VA at the onset of treatment was normal to moderate, 4 (20%) experienced worsening of their VA. Among the 9 people who initially presented with severely decreased VA, 3 (33%) experienced a further decline in their vision (
Age
Optic nerve sheath meningiomas in patients > 45 years old had higher rates of vision deterioration compared with younger patients (41% vs 8%). Also, 1 out of 14 patients treated at age < 46 years worsened in terms of overall vision status compared with 6 out of 15 patients treated at age ≥
No significant association was noted between total radiation dose (
Discussion
There are currently no established consensus guidelines for the management of ONSMs specifically, although the National Comprehensive Cancer Network (NCCN) provides recommendations for the treatment of meningiomas in general and stresses the importance of early intervention to preserve visual function in cases involving the optic nerve.13 In most cases, observation is the preferred approach for asymptomatic or minimally symptomatic patients, while RT is reserved for those with impending vision loss or in cases of progressive or advanced diseases.14 Since ONSM progression can be unpredictable, it is crucial to ensure that any intervention prioritizes the maintenance or improvement of vision.15
Before the development of our proposed system, there was no standardization of visual outcomes following treatment for optic nerve tumors. To address this gap, we have created the WEVO classification system, which we applied to our cohort of 29 closely monitored ONSM cases. Our analyses also aimed to identify any factors within our cohort that may predict a worsened outcome and provide guidance to help maintain or improve vision.
Our patient cohort is consistent with those of other limited series and is highly representative of the ONSM population, thereby increasing the generalizability of our results and making them suitable for future meta-analyses. In their largest known retrospective cohort study of visual outcomes for ONSMs, Dutton conducted a review of nearly 500 cases and characterized ONSMs.1 The patients in the study were mostly middle-aged women (with a mean age of 47 y) and 5% had bilateral tumors. Additionally, 25% of patients had a VA of counting fingers or worse, while 45% had a VA of 20/40 or better.1 In our cohort, the median age was 47 years and the mean age was 48.9 years. Of the 29 patients, 83% werewomen, 1 had bilateral disease (3.7%), 24% presented with severe visual deficits, and 45% presented with a VA of 20/40 or better.
Our series of 29 cases had well-documented, comprehensive long-term radiographic and ophthalmologic follow-up, with a median follow-up of 81 months and a mean of 93 months (range, 17-240 mo). To the best of our knowledge, this study has the longest median and most comprehensive visual and radiographic follow-up of primary ONSMs following FSRT in the medical literature. Vanikieti et al reported a cohort of 34 patients with ONSM with an impressive visual examination range of follow-up of 6 to 251 months; however, only VF and VA were reported and the overall visual function was defined as strictly related to VF and VA. They did not have full radiographic follow-up for their entire cohort.16 Most studies had similarly small samples (10-45 patients), with shorter follow-up times ranging from a median of 54 weeks to 5 years.8,17-19 Two studies, Smee et al and Metellus et al, had median follow-ups of 86 months and 90 months, respectively, but had only 15 and 9 patients.20,21 Paulsen et al followed 109 patients after FSRT; however, long-term ophthalmologic and radiographic outcomes were available for only 38% and 33% of all patients, respectively, and 67% were “secondary” ONSMs.22 Turbin et al had a mean follow-up of 150.2 months with a range of 51-516 months (SD, 74.7 mo) and followed 64 patients, only 16 of whom had radiation alone and another 16 of whom had RT plus surgery.5 It is important to note that most studies only reported VA as the measure of visual outcome. With our study’s long-term median follow-up of more than 7 years and complete visual outcomes, we were uniquely able to work with ocular oncology at the Wills Eye Institute to develop the WEVO criteria to define visual outcomes for each case.
Multiple studies have attempted to identify predictive factors for outcomes of RT to best stratify patients. Kennerdell et al reported worsened visual outcomes in those with a VA of 20/40 or below or with a constricting VF, although in this study, VA alone was reported in only 9 patients.10 Similarly, Saeed et al reported worsened visual outcomes in patients with pretreatment VA below 20/50.23,24 Neither study considered integrated VA, VF, and CV to determine whether visual outcomes improved, remained stable, or worsened, nor did they individually assess pretreatment VF or CV as predictors for visual outcome. Our findings suggested that VF and CV defects, rather than VA, were more predictive of worsened visual outcomes using the WEVO criteria. Our study also noted that patients below age 46 were more likely to have improved or unchanged vision compared with patients at or older than 46 years. Perhaps related to younger patients having better outcomes, Wright et al reported a more active primary ONSM in patients < 40 years old and recommended more active treatment, such as surgery, assuming more active meningothelial cells.25 Paulsen et al found that a radiation dose of 54 Gy vs < 54 Gy was predictive of radiographic control. They also determined that sex, histology (biopsy taken vs not taken), early RT vs treatment at progression, and tumor size < 5 cm3 vs > 5 cm3 were not predictive of VA, ocular motility, VF, or tumor control following RT.22 Similarly, our study did not find a significant impact from tumor size or time of radiation with respect to visual outcome. Unique to our study is the significance of pretreatment for older age, VF, and CV defects rather than VA or TTT in regard to poor visual outcomes. More recently, in a cohort of 43 patients treated between 2015 and 2021, who underwent external beam radiation therapy (EBRT) for ONSMs, Tang et al observed that patients with severe vision loss at diagnosis or a duration of vision loss exceeding 12 months had a lower likelihood of vision recovery after treatment.26 This was similar to our findings; however, using our WEVO criteria, we did note that the 6-month duration of vision loss was sufficient to predict worsening visual outcomes after treatment.
Several limitations to our study should be considered. Most importantly, our study was retrospective in design and limited to a single institution. Selection bias may play an important role in the outcomes as there is an urgency to treat patients who present with severe visual loss vs those with mild loss. The overall sample size is relatively limited due to the rarity of the disease (2%-3% of all meningiomas), although our study is similar in size to many published series. Another important limitation is that several radiation treatment parameters were not readily available from paper chart extraction (eg, MDPD was available for 26 cases only). Nevertheless, we felt that all patients who had total dose and fractionation as well as a clearly documented TTT from diagnosis were appropriate to include in our study. Strengths of our study include length of follow-up, inclusion of only primary ONSMs, as well as comprehensive ophthalmologic and radiographic evaluation.
Conclusions
Visual outcomes are of great importance to consider in the treatment strategy and patient discussion surrounding ONSMs. This study, along with others, adds to the literature supporting the efficacy and durability of FSRT for ONSMs with respect to local control and visual preservation.17,22,27 We propose a classification that defines a comprehensive visual outcome endpoint based on the WEVO criteria as improved, worsened, or unchanged. Using these criteria, we found that age at treatment, CV defect, and large VF defect were associated with poor visual outcomes. However, we did not observe any correlation between VA, radiation dose statistics (total dose, maximum dose to the optic nerve and the tumor, and MDPD), lesion size, and the ultimate visual outcomes. In the future, we recommend using the WEVO classification to further contribute to studies that can predict visual outcomes and lead to decisions that preserve vision for patients with ONSM.
References
- Dutton JJ Optic nerve sheath meningiomas. Surv Ophthalmol. 1992; 3(37):167-183
- Spencer WH Primary neoplasms of the optic nerve and its sheaths: clinical features and current concepts of pathogenetic mechanisms. Trans Am Ophthalmol Soc. 1972; undefined(70):490-528
- Carrasco JR, Penne RB Optic nerve sheath meningiomas and advanced treatment options. Curr Opin Ophthalmol. 2004; 5(15):406-410
- Fineman MS, Augsburger JJ A new approach to an old problem. Surv Ophthalmol. 1999; 6(43):519-524
- Turbin RE, Thompson CR, Kennerdell JS, Cockerham KP, Kupersmith MJ A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology. 2002; 5(109):890-899
- Turbin RE, Pokorny K Diagnosis and treatment of orbital optic nerve sheath meningioma. Cancer Control. 2004; 5(11):334-341
- Egan RA, Lessell S A contribution to the natural history of optic nerve sheath meningiomas. Arch Ophthalmol. 2002; 11(120):1505-1508
- Pitz S, Becker G, Schiefer U Stereotactic fractionated irradiation of optic nerve sheath meningioma: a new treatment alternative. Br J Ophthalmol. 2002; 11(86):1265-1268
- Miller NR New concepts in the diagnosis and management of optic nerve sheath meningioma. J Neuroophthalmol. 2006; 3(26):200-208
- Kennerdell JS, Maroon JC, Malton M, Warren FA The management of optic nerve sheath meningiomas. Am J Ophthalmol. 1988; 4(106):450-457
- Shapey J, Sabin HI, Danesh-Meyer HV, Kaye AH Diagnosis and management of optic nerve sheath meningiomas. J Clin Neurosci. 2013; 8(20):1045-1056
- Colenbrander A undefined. Visual standards, aspects and ranges of vision loss with emphasis on population surveys. 2002; undefined(undefined):undefined-undefined
- Nath SK, Trifiletti DM, Zaorsky NG, Rusthoven CG Central nervous system cancers. Absol Clin Radiat Oncol Rev. 2019; undefined(undefined):83-131
- Falardeau J undefined. Current Diagnosis and Management of Optic Nerve Sheath Meningioma - American Academy of Ophthalmology. 2007; undefined(undefined):undefined-undefined
- Egan RA, Lessell S A contribution to the natural history of optic nerve sheath meningiomas. Arch Ophthalmol. 2002; 11(120):1505-1508
- Vanikieti K, Chaiwithooanukul C, Puataweepong P, Jindahra P, Padungkiatsagul T Long-term visual function after fractionated stereotactic radiotherapy for primary optic nerve sheath meningioma: a retrospective analysis of 34 subjects. Clin Ophthalmol. 2022; undefined(16):3119-3128
- Andrews DW, Faroozan R, Yang BP Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery. 2002; 4(51):890-902
- Narayan S, Cornblath WT, Sandler HM, Elner V, Hayman JA Preliminary visual outcomes after three-dimensional Conformal radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2003; 2(56):537-543
- Becker G, Jeremic B, Pitz S Stereotactic fractionated radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2002; 5(54):1422-1429
- Smee RI, Schneider M, Williams JR Optic nerve sheath meningiomas—non-surgical treatment. Clin Oncol (R Coll Radiol). 2009; 1(21):8-13
- Metellus P, Kapoor S, Kharkar S Fractionated Conformal radiotherapy for management of optic nerve sheath Meningiomas: long-term outcomes of tumor control and visual function at a single institution. Int J Radiat Oncol Biol Phys. 2011; 1(80):185-192
- Paulsen F, Doerr S, Wilhelm H Fractionated stereotactic radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2012; 2(82):773-778
- Kim JW, Rizzo JF, Lessell S Controversies in the management of optic nerve sheath meningiomas. Int Ophthalmol Clin. 2005; 4(45):15-23
- Saeed P, Rootman J, Nugent RA, White VA, Mackenzie IR, Koornneef L Optic nerve sheath meningiomas. Ophthalmology. 2003; 10(110):2019-2030
- Wright JE, McNab AA, McDonald WI Primary optic nerve sheath meningioma. Br J Ophthalmol. 1989; 12(73):960-966
- Tang T, Wang J, Lin T, Zhai Z, Song X The treatment efficacy of radiotherapy for optic nerve sheath meningioma. Eye (Lond). 2023; undefined(undefined):undefined-undefined
- Hamilton S, Nichol A, Hsu F Visual outcomes and local control after fractionated stereotactic radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2015; 3(93):E63-E64
Citation
Dastgheyb S, Fernandez C, Werner-Wasik M, Farrell C, Bilyk J, Shields C, Sergott RC, Shi W. A Novel Framework to Define and Prognosticate Visual Outcomes Following Fractionated Radiation Therapy for Optic Nerve Sheath Meningiomas. Appl Radiat Oncol. 2023;(3):25-33.
doi:10.37549/ARO-D-23-00019
September 1, 2023