A Rare Case of Sweat Gland Carcinoma of the Breast
Affiliations
- Medicover Cancer Institute, Hyderabad, Telangana
Abstract
Sweat gland carcinoma of the skin is a rare malignancy. Malignant sweat gland carcinoma of the breast is among one of the rarer sites of sweat gland malignancies, making it a very rare entity. with few cases reported in the literature. Sweat gland malignant lesions can arise in eccrine or apocrine sweat glands present in the skin. Here we report a case of malignant sweat gland carcinoma of the breast with axillary nodal metastasis treated with surgery (modified radical mastectomy) followed by adjuvant radiation therapy in view of lymph node metastasis.
Case Summary
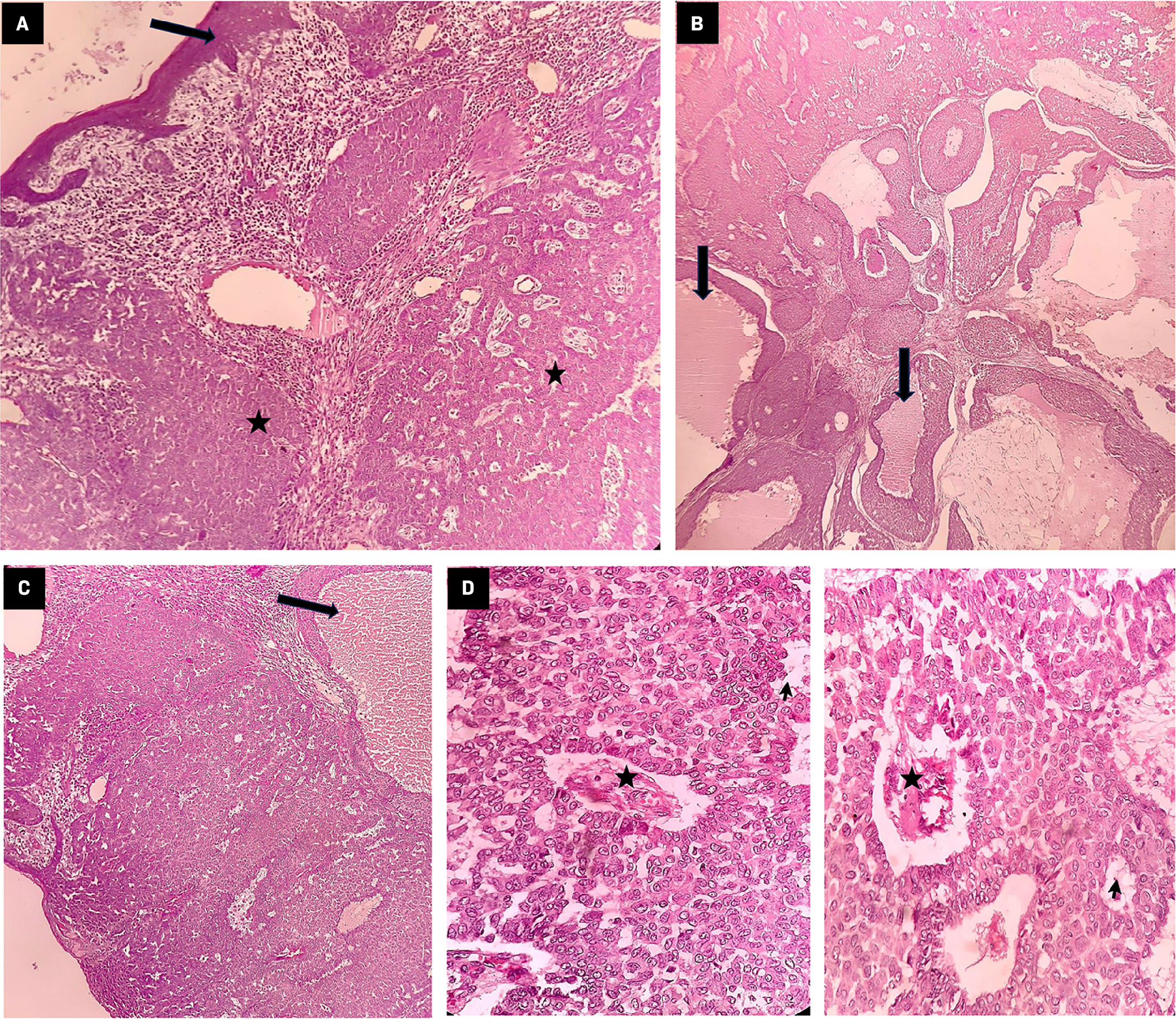
The patient is a 68-year-old woman with no associated medical comorbidities and no family history of malignancy. She had presented with an ulcerated fungating right breast mass lesion in July 2021. She had noticed this lesion first in 2014. Over 7 years, the lesion had gradually progressed to the present size. She was initially evaluated by a surgical oncologist in July 2021. Clinical examination revealed an ulcerated right breast mass lesion measuring 10 × 10 cm involving the central part of the breast. It also showed that the nipple-areola complex appeared involved, the lesion was mobile and not fixed to the chest wall, palpable right axillary lymph nodes, and no clinical evidence of palpable supraclavicular nodes. Clinical examination of the opposite breast was normal. Breast lesion tissue biopsy showed features of malignant sweat gland carcinoma. A staging workup with ultrasound of the abdomen and pelvis and a CT scan of the chest showed no evidence of distant metastasis. The patient underwent modified radical mastectomy with axillary lymph node dissection in August 2021. Histopathology showed ( Figure 1 ) features suggestive of high-grade malignant sweat gland carcinoma (skin adnexal tumor), a 10 × 7-cm tumor, no ductal carcinoma in situ, lymphovascular invasion (LVSI), nipple and areola involvement, skin involvement, tumor-free resected margins, and axillary lymph nodes that were 3/18 positive for metastasis with the pathological stage pT3 N2 as per the TNM (tumor, node, mestastasis) 8th edition for cutaneous adnexal tumors.
Section shows tumor-infiltrating dermis (star) arranged in nests and a glandular pattern with overlying intact epidermis (arrow) (hematoxylin and eosin [H&E] × 40) (A). Tumor shows the formation of duct-like structures (arrows) filled with secretions suggestive of sweat gland differentiation (H&E × 40, H&E × 100) (B). Tumor shows the formation of duct-like structures (arrows) filled with secretions suggestive of sweat gland differentiation (H&E × 100) (C). Both figures: tumor is composed of round to oval cells arranged in solid, papillary (star) and tubular (arrow) patterns (H&E × 400) (D).
In view of the locally advanced nature of the primary tumor and presence of high-risk pathological features (large tumor size, LVSI, and nodal involvement), the patient received adjuvant locoregional radiation therapy ( Figure 2 ). The internal mammary node region was not included in the field of radiation as there was no radiological evidence of disease or data showing any significant benefit to using this treatment for sweat gland carcinoma of the breast.
Radiation therapy treatment planning simulation CT image and primary treatment volume contour as visible ondigitally reconstructed radiographs (A, B). Radiation therapy dose distribution in color wash covering the chest wall, axilla, and supraclavicular nodal region (C). Electron-beam radiation therapy plan showing dose distribution covering the chest wall scar and drain sites (D).
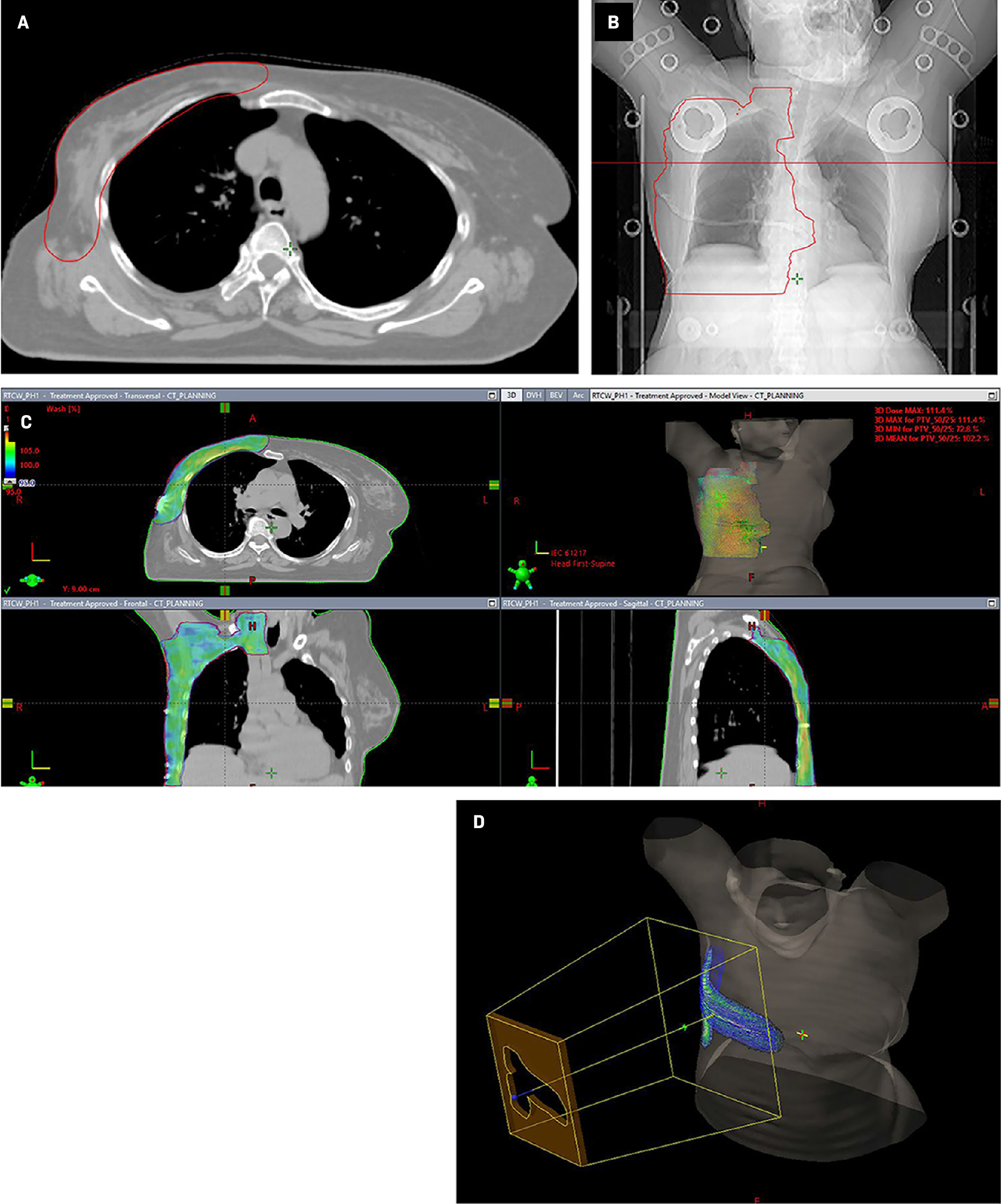
Radiation therapy dose prescription:
Phase 1: Planning treatment volume (PTV), which includes the chest wall, axilla, and supraclavicular nodal region, 50 Gy in 25 fractions, 2 Gy per fraction, with the TrueBeam (Varian) linear accelerator using 6 MV photons with the RapidArc technique. A 5-mm gel bolus material was used to cover the chest wall target region (including the surgical scar and drain site) to have adequate radiation dose buildup over the skin of the chest wall.
Phase 2: PTV boost, which includes the chest wall surgical scar and drain site, of 10 Gy in 5 fractions, 2 Gy per fraction with 6 MeV electrons.
The patient tolerated the treatment well with grade 3 skin reactions, which recovered well by her first follow-up at 1 month. The patient was disease free as per the clinical examination and imaging (PET/CT) at the 1-year follow-up.
Diagnosis
Sweat gland carcinoma of the breast. The differential diagnosis includes other common cutaneous cancers such as basal cell carcinoma, squamous cell carcinoma, or metastatic carcinoma of the skin. Other possible differentials include hidradenoma and primary ductal breast malignancy.
Discussion
Sweat gland malignant lesions are a rare entity in clinical practice. The reported incidence in the literature is around 0.005% of all tumor specimens resected surgically.1 These malignant lesions can arise from 2 anatomically different types of sweat glands in the skin: apocrine or eccrine. The main difference between apocrine and eccrine sweat glands is that the secretions of the apocrine sweat glands are viscid, whereas the secretions of eccrine sweat glands are watery. Furthermore, apocrine sweat glands are always connected to hair follicles while eccrine sweat glands are not. Apocrine sweat glands are predominantly present in the axillary and perianal region, whereas eccrine sweat glands are present all over the skin. The breast (mammary gland) is also considered a modified apocrine sweat gland. Sweat gland carcinoma arises from skin appendages in the dermis.2 Exposure to ultraviolet rays has been suggested as an etiological factor.3 Immune suppression has also been considered an etiological factor for development of eccrine carcinoma.4
Sweat gland carcinomas occur primarily in adults, with peak incidence in the fifth and sixth decades of life.5 - 7 The majority occur in the genital skin and perineum (34.5%), followed by the trunk (26.4%), head and neck (18.3%), and lower extremities (13.9%).5, 6, 8, 9 The reported incidence is the same in men and women.10 Tumors usually present as a solitary nodule or a plaque on the skin, and a history of rapid growth of the lesion suggests malignancy. These lesions have the tendency to infiltrate locally and also have regional nodal or sometimes distant metastasis.11 Malignant sweat gland carcinoma of the breast is a rare site for a sweat gland malignancy, making it a very rare entity with few cases reported in the literature.
Histological evaluation, along with immunohistochemistry, is important in distinguishing these lesions from breast parenchymal malignant lesions. Microscopically they have the appearance of an adenocarcinoma with well-developed glandular lumina, showing characteristic evidence of “decapitation secretion.”12 Tumor cells are PAS (periodic acid-Schiff)-positive due to glycogen granules; they are also diastase resistant.5 Other features include a glandular lumen, which may be narrow or slightly dilated with the cells of glandular and papillary structures being large, with a strongly eosinophilic cytoplasm containing hemosiderin.12 Sudan stain for lipids may be either negative or positive with mucin often present in and around the lumen of the glandular structures.12
Because malignant sweat gland carcinoma of the breast is rare, no standard management guidelines exist. Surgery is the preferred upfront treatment for nonmetastatic operable lesions. For primary apocrine sweat gland carcinoma, wide local excision with regional lymph node dissection is considered for clinically node-positive disease.3 For clinically node-negative disease, prophylactic lymph node dissection remains controversial.3 Some studies have shown that prophylactic lymph node dissection has no influence on survival in these patients.12, 13 However, several case reports have found that sentinel lymph node biopsy is useful in these patients.14, 15 The local recurrence rate is high after surgery,16 and the role of postoperative adjuvant treatment remains unclear.3 Because weat gland carcinoma is generally considered resistant to chemotherapy, adjuvant chemotherapy is not routinely recommended.17, 18 However, in metastatic setting, chemotherapy has shown favorable responses.19, 20 Radiation therapy has a role in adjuvant setting as it reduces the risk of relapse.18 It has been suggested that adjuvant radiation therapy should be considered if the disease has 1 or more risk factors such as T size of 5 cm or more, positive margins, a moderate to poorly differentiated tumor, or LVSI.18 Prognostic factors for sweat gland carcinoma are difficult to identify owing to the small number of reported cases. The likely prognostic factors include tumor size, histological type, lymph node involvement, and distant metastasis.11 A 10-year disease-free survival rate of 56% in the absence of lymph node metastasis is observed, which falls to 9% if nodes are involved.5 In the largest retrospective cohort study, the median overall survival and 5-year disease-specific survival rates were 51.5 months and 88%, respectively.21
Conclusion
Malignant sweat gland carcinoma of the breast is a rare entity in clinical practice. These tumors are locally invasive and have a predilection for early nodal metastasis and, rarely, distant metastasis. Curative therapy involves upfront surgery for operable cases. Currently, there is a lack of specific management guidelines for adjuvant therapy. Adjuvant radiation therapy should be considered in locoregionally advanced cases with high-risk pathological features for better local control. Currently, there is no significant role of adjuvant chemotherapy for malignant sweat gland carcinoma of the breast.
References
Citation
MA A, M B, SS M, P M, LM P, PK S, M S, YSB S, O M. A Rare Case of Sweat Gland Carcinoma of the Breast. Appl Radiat Oncol. 2024;(2):32 - 36.
doi:10.37549/ARO-D-24-00005
June 1, 2024