A review of the role of external-beam radiation therapy in nonmelanomatous skin cancer
Images

SAM-CME credits available here
Nonmelanomatous skin cancers (NMSC), specifically basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), are the most common malignancies in the United States. Primarily managed surgically, these malignancies are associated with excellent prognosis, with a 1% to 5% rate of disease recurrence after complete excision, and exceedingly rare instances of distant metastases (1% to 3%).1 Historically, radiation therapy (RT) has played a prominent role in definitive management as an alternative to surgery, particularly in cosmetically sensitive areas. Improvements in surgical techniques over recent decades and the widespread use of Mohs micrographic surgery (MMS) has led to a decline in the use of curative RT for skin cancers. However, it continues to be commonly used for patients who are poor surgical candidates, have larger lesions in cosmetically sensitive regions of the face, or in the postoperative setting for tumors with high-risk pathologic features. Finally, RT offers excellent symptom palliation in patients with incurable disease. Advanced treatment techniques (electronic brachytherapy with miniature X-ray tube [Xoft; San Jose, California] and high-dose rate brachytherapy) are evolving with encouraging results, but are beyond the scope of this article. This article reviews common indications, dosing, techniques, and outcomes for external-beam RT for NMSC.
Indications for Definitive RT for NMSC
Both surgery and RT provide excellent cure rates for early stage NMSC; however, surgery is the preferred method of management, as it can be performed in a single session and may be associated with superior oncologic and cosmetic outcomes. A randomized study of 347 patients with < 4 cm BCC of the face compared outcomes between MMS and definitive RT, and determined a local failure rate of 0.7% with MMS, and 7.5% with RT. Additionally, the cosmetic outcome was rated “good” more often with MMS (87% vs. 69%).2 The quality of the comparison may have been compromised by uncontrolled technique of RT (55% received interstitial brachytherapy and 45% received orthovoltage therapy). As the only randomized study, this trial remains crucial in guiding medical decision-making. Definitive RT is typically contraindicated for large tumors with bone invasion, nodal metastases, and previously irradiated recurrent tumors. RT should also be avoided in patients with genetic syndromes associated with increased radiosensitivity (xeroderma pigmentosum and basal cell nevus syndrome) and active connective tissue diseases (scleroderma and systemic lupus erythematous).3
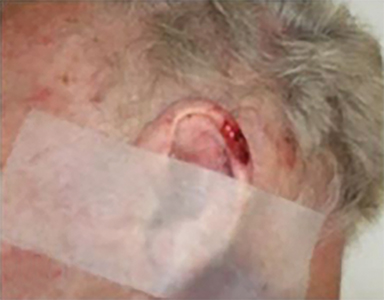
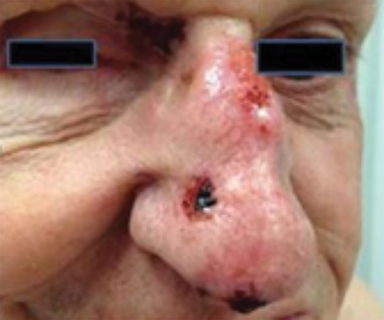
Optimal candidates for definitive RT include elderly patients with comorbidities; unresectable disease; and lesions involving the eyelid, external ear (Figure 1), nose (Figure 2), canthi of the eye (Figure 2), brow, or lip, which may result in significant cosmetic or functional deficits from surgery.4 Much of the data supporting the safety and efficacy of definitive RT in these patients is older, when its use was more common. A review of 986 BCC and SCC of the skin overlying the eyelid treated with definitive RT yielded a 5-year cure rate of 96.4%.5 Similarly, an excellent local control rate was observed in a review of 334 BCC and SCC of the external ear at the Princess Margaret Hospital treated with definitive RT, with a 2-year local control rate of 87% and severe late toxicity of 7% of patients.6 The recently approved hedgehog pathway inhibitor, vismodegib, demonstrated encouraging response rates in unresectable BCC, and may become first-line therapy with additional clinical experience.7 Our practice has shifted toward upfront vismodegib for large BCC, with RT reserved for poor responders.
While definitive RT can provide acceptable tumor control for T1-3N0 NMSC, inferior outcomes are observed for T4 tumors and nodal metastases. A local control rate of just 53% at 5 years was reported in patients with T4 BCC and SCC treated with definitive RT.8,9 Recurrent disease (p < 0.01), bone involvement (p < 0.01), and perineural invasion (PNI) (p < 0.01) are associated with significantly worse local control and cause-specific survival with definitive RT. Patients with nodal metastases have locoregional recurrence rates (LRR) of 30% to 50% and cancer-related mortality as high as 30% with definitive RT.10 These suboptimal outcomes highlight the need for intensifying treatment with multimodality therapy, including surgery and postoperative RT for patients with advanced disease.
Radiation Targeting and Doses for Definitive RT for NMSC
The dose and fractionation for definitive RT is primarily driven by proximity to normal tissues, cosmetic impact, and patient tolerance and convenience. Overall, definitive doses ranging from 45-80 Gy have demonstrated satisfactory cosmetic outcomes, with hypopigmentation (91.8%) and telangiectasia (82.2%) as the most common cosmetic change 4 years after RT.11
A radial margin of 1-2 cm is typically used, while smaller margins are appropriate for well-circumscribed lesions and larger margins for infiltrative lesions. Careful assessment of depth using 3-dimensional planning to ensure adequate coverage is crucial. Per the American College of Radiology Appropriateness criteria, conventionally fractionated regimens for definitive RT include 70 Gy/35 fractions and 60 Gy/30 fractions. Moderately hypofractionated courses include 55 Gy/20 fractions or 50 Gy/15 fractions. Extreme hypofractionation of 40 Gy/5 fractions (2-3 fractions weekly) or 20 Gy/2 fractions weekly can be considered in elderly or poorly performing patients.4
Indications for Postoperative RT for NMSC
BCC is rarely treated with postoperative RT, as it is typically associated with an exceedingly low risk of recurrence after surgery alone. Patients with positive margin, focal cartilage invasion, or PNI are often still candidates for closer observation with re-resection for salvage, if necessary.1,12 Postoperative RT for BCC should be considered for persistently positive margins after multiple resections, T4 disease with extensive bone and soft tissue invasion, lymph node (LN) metastasis, or clinical PNI.13
SCC with high-risk features is associated with high rates of local recurrence from 20% to 50% with surgery alone, and postoperative RT is recommended to optimize locoregional control. Patients with T4 disease, positive margin, clinical PNI, or patients with 2 or more intermediate risk factors, including tumor > 2 cm, poorly differentiation, depth > 4 mm or beyond subcutaneous fat, desmoplastic growth pattern, recurrent tumor, ear and hair-bearing lip, microscopic PNI, lymphovascular space invasion (LSVI) and immunosuppressed status (IS) should be considered for postoperative RT.14
PNI, while not common (5% to 10% of SCC), is an important risk factor for local recurrence, as well as regional and distant metastases. Clinical PNI is defined by neurologic manifestations, most commonly involving the trigeminal or facial nerves, or radiographic nerve enhancement.15,16 Microscopic PNI is appreciated histologically in an asymptomatic patient. The presence of clinical PNI is associated with significantly lower rates of 5-year local control (57% vs. 90%; p ≤ 0.001) and overall survival (57% vs. 69%; p = 0.03) compared to microscopic PNI in patients treated aggressively with surgery and postoperative RT.13 Given inferior outcomes, RT is always recommended in cases of clinical PNI; however, the role of postoperative RT in the setting of microscopic PNI is less clear. Lin et al demonstrated improved relapse-free survival with focal vs. extensive microscopic PNI (86% vs. 74%; p = 0.1), but unfortunately the distinction between focal and extensive was not quantified.16 Postoperative RT is recommended for microscopic PNI if multifocal, diameter of nerve > 0.1 mm, named nerves, or IS, as these factors are associated with higher local recurrence rates.17,18 Postoperative RT may be deferred in immunocompetent patients with nonrecurrent disease, with 1 or 2 isolated areas of microscopic PNI in unnamed nerves, with a diameter of < 0.1 mm.
PNI may also be associated with increased nodal failure and its presence in combination with primary sites with a high propensity for LN metastases (cheek, ear, nasal skin) should prompt consideration for elective nodal coverage. Lin et al demonstrated that patients who developed recurrent disease with pathologic PNI had a significantly increased risk of regional recurrence (26% vs. 5%; p = 0.02).16 Patients with advanced T stage, recurrent primary tumors LVSI, and IS are also at significantly higher risk for LN metastases, ranging from 29% to 50%.10,19-21
In patients with clinically involved LNs, a therapeutic lymph node dissection (LND) followed by postoperative RT is the current standard of care. LRR after LND alone is 11% to 38%, and even after multimodality therapy, 5-year disease-free survival is 60% to 70%. Independent predictors for worse survival include increased nodal size ≥ 3 cm, multiple LNs, extracapsular extension (ECE), incomplete dissection, and surgery monotherapy.20-22 A review of 167 patients with SCC metastatic to the parotid or cervical LNs demonstrated significantly lower rates of LRR (20% vs. 43%) and higher 5-year disease-free (73% vs. 54%; p = 0.004) and overall survival (66% vs. 27%; p = .003) with surgery and postoperative RT compared to surgery alone.20 Similar to mucosal SCC of the head and neck, RT can be avoided after LND in immunocompetent patients with a single LN, < 3 cm, without ECE, as regional recurrence is < 5%.23
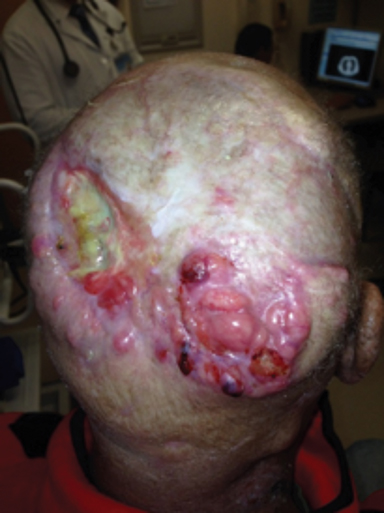
Chronic immunosuppression in solid organ transplant recipients (OTR) or in patients with chronic lymphocytic leukemia (CLL) is associated with up to 100-fold higher incidence of NMSC and tend to have more high-risk features of PNI, LVSI, infiltrative, head and neck location, and nodal metastasis (Figure 3).1 These patients have significantly worse disease outcomes, and skin cancer may even contribute to 5% to 10% of mortality.24-26 Manyam et al demonstrated that immunosuppressed patients treated with surgery and postoperative RT had significantly worse 2-year locoregional recurrence-free survival (47% vs. 86%; p < 0.001) and progression-free survival (39% vs. 72%; p = 0.002) compared to immunocompetent patients, and IS status was significantly associated with increased LRR (HR 3.79; p < 0.0001) on multivariate analysis.24 Postoperative RT should be strongly considered for this population, even in early stage disease. The benefit of intensifying therapy with earlier initiation of RT, dose escalation, or concurrent systemic therapy requires future prospective study. Immunosuppressive regimens in OTR are an important consideration, and transitioning of agents should be discussed with the patient and transplant physician after a new diagnosis of SCC. Phase III data has demonstrated a significantly decreased incidence in development of new SCC (22% vs. 39%; p = 0.02) with sirolimus, compared to tacrolimus.27
Appropriate prognostication using the current AJCC skin cancer staging is challenging given that T2 tumors represent an extremely heterogeneous population of patients with varying outcomes. Improving granularity within staging categories is important to better understand outcomes and treatment recommendations. The Brigham and Women’s Hospital revised skin cancer staging system defined high-risk features of poor differentiation, tumor diameter ≥ 2 cm, PNI ≥ 0.1 mm, or tumor invasion beyond fat (excluding bone invasion, which upgrades to T3), and created a T2a (1 high-risk feature) and T2b category (2-3 high-risk features) category, which was shown to be a more effective prognostic tool.28 However, the absence of IS status within this staging system may represent a potential area of deficiency, and should be accounted for in prognostic systems.
Radiation Targeting and Doses for Adjuvant RT for NMSC
Common postoperative regimens for the head and neck include 60 Gy in 30 fractions and 50 Gy in 20 fractions with negative margins or no ECE, and 66 Gy in 33 fractions and 55 Gy in 20 fractions with positive margins or ECE. For axilla or inguinal LNs with no ECE, 45-50 Gy in 25 fractions is used and 60-66 Gy in 30-33 fractions is used with ECE.
Typically, the parotid and levels IB-V nodes are at risk for NMSC of the head and neck, although coverage of lymphatics heavily depends on the location of the primary. Inclusion of facial lymphatics should be considered for T3 and T4 disease, typically of the forehead, scalp, cheek, medial canthus, and nose, or in the presence of multiple high-risk features. For NMSC of the extremities and trunk, coverage of lymphatics depends on the location of the primary and surgical evaluation. The clinical target volume for irradiation of clinical PNI should include the involved nerve, portion of the nerve proximally at the skull base, the distal skin innervated by the nerve, major communicating branches, and the compartment in which the nerve is located.29
Role of Concurrent Systemic Therapy with RT for NMSC
Vismodegib is the first approved systemic therapy for advanced BCC and is indicated in the recurrent, inoperable setting or in the metastatic setting. A phase II study of patients with inoperable or metastatic BCC treated with vismodegib demonstrated response rates of 43% (95% CI, 31-56; p < 0.0001) and 30% (95%, CI 16-48; p = 0.0001), respectively, with a serious adverse event rate of 25%.7 Mylagias and fatigue can be dose-limiting toxicities, which impair continuation of therapy in some patients. Recent evidence suggests that alternative dosing strategies improve the tolerability profile without compromising efficacy.30 Future practice may be guided by studies investigating the addition of vismodegib to RT in very high-risk BCC.31
Currently, no prospective randomized evidence evaluates the benefit of concurrent systemic therapy with definitive or postoperative RT for high-risk SCC. The decision to include concurrent systemic therapy in the postoperative setting is extrapolated from literature in head and neck mucosal SCC. These trials demonstrated significantly improved locoregional control and progression-free survival with concurrent chemotherapy, and further analysis demonstrated that the benefit is limited to positive margins and ECE.23,32,33 The addition of concurrent cisplatin to postoperative RT should be considered for ECE, positive margins, or with definitive RT for patients with unresectable disease.
Epidermal growth factor receptor (EGFR) inhibitors have gained interest as monotherapy and in combination with surgery and/or RT for SCC. A phase II study of neoadjuvant gefitinib followed by surgery, RT, or both in 22 patients with locally advanced SCC demonstrated a complete response rate of 18%, partial response rate of 27%, and 2-year progression-free survival of 60%.34 Similarly, a phase II study of cetuximab monotherapy for unresectable or metastatic SCC demonstrated a 30% response rate and 70% disease stabilization rate.35 No available data investigates the use of EGFR inhibitors concurrently with RT for cutaneous SCC in the definitive or postoperative setting, but it can be considered in elderly patients or patients with renal disease who are not candidates for cisplatin. More recently, checkpoint inhibitors have shown preliminary promise in metastatic mucosal and cutaneous SCC, and ongoing studies will further clarify the role of immunotherapy.36,37
Conclusion
Radiation therapy plays an important role in both the definitive and postoperative management of NMSC, especially in patients with high-risk disease. Chronic immune suppression represents a high-risk population with significantly inferior outcomes and its presence should be incorporated into clinical decision-making and multidisciplinary management. Improvements should be made in the current prognostication systems to better represent and categorize high-risk disease. Treatment paradigms will evolve with the continued development of novel systemic therapies in both BCC and SCC.
References
- Lott DG, Manz R, Koch C, Lorenz RR. Aggressive behavior of nonmelanotic skin cancers in solid organ transplant recipients. Transplantation. 2010;90(6):683-687.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? Results of a randomized study. Br J Cancer. 1997;76(1):100-106.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Squamous Cell Skin Cancer. 2016.
- Koyfman SA, Cooper JS, Beitler JJ, et al. ACR Appropriateness Criteria Aggressive Nonmelanomatous Skin Cancer of the Head and Neck. Head Neck. 2016;38(2):175-182.
- Caccialanza M, Piccinno R, Gaiani F, Contini D. Relevance of dermatologic radiotherapy in the therapeutic strategy of skin epithelial neoplasms: excellent results in the treatment of lesions localized on eyelids and skin overlying the cartilage of the nose. G Ital Dermatol Venereol. 2013;148(1):83-88.
- Silva JJ, Tsang RW, Panzarella T, et al. Results of radiotherapy for epithelial skin cancer of the pinna: the Princess Margaret Hospital experience, 1982-1993. Int J Radiat Oncol Biol Phys. 2000;47(2):451-459.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23): 2171-2179.
- Lee WR, Mendenhall WM, Parsons JT, Million RR. Radical radiotherapy for T4 carcinoma of the skin of the head and neck: a multivariate analysis. Head Neck. Jul-Aug 1993;15(4):320-324.
- Al-Othman MO, Mendenhall WM, Amdur RJ. Radiotherapy alone for clinical T4 skin carcinoma of the head and neck with surgery reserved for salvage. Am J Otolaryngol. 2001;22(6):387-390.
- Kwan W, Wilson D, Moravan V. Radiotherapy for locally advanced basal cell and squamous cell carcinomas of the skin. Int J Radiat Oncol Biol Phys. 2004;60(2):406-411.
- Rupprecht R, Lippold A, Auras C, et al. Late side-effects with cosmetic relevance following soft X-ray therapy of cutaneous neoplasias. J Eur Acad Dermatol Venereol. 2007;21(2):178-185.
- Traywick C, O’Reilly FM. Management of skin cancer in solid organ transplant recipients. Dermatol Therapy. 2005;18(1):12-18.
- Jackson JE, Dickie GJ, Wiltshire KL, et al. Radiotherapy for perineural invasion in cutaneous head and neck carcinomas: toward a risk-adapted treatment approach. Head Neck. 2009;31(5):604-610.
- Jennings L, Schmults CD. Management of high-risk cutaneous squamous cell carcinoma. J Clin Aesthet Dermatol. 2010;3(4):39-48.
- Mendenhall WM, Amdur RJ, Hinerman RW, et al. Radiotherapy for cutaneous squamous and basal cell carcinomas of the head and neck. Laryngoscope. 2009;119(10):1994-1999.
- Lin C, Tripcony L, Keller J, et al. Perineural infiltration of cutaneous squamous cell carcinoma and basal cell carcinoma without clinical features. Int J Radiat Oncol Biol Phys. 2012;82(1):334-340.
- Han A, Ratner D. What is the role of adjuvant radiotherapy in the treatment of cutaneous squamous cell carcinoma with perineural invasion? Cancer. 2007;109(6):1053-1059.
- National Comprehensive Cancer N. Clinical Practice Guidelines in Oncology Skin Cancer. 3/29/2017 2017. https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf.
- Wang JT, Palme CE, Morgan GJ, et al. Predictors of outcome in patients with metastatic cutaneous head and neck squamous cell carcinoma involving cervical lymph nodes: Improved survival with the addition of adjuvant radiotherapy. Head Neck. 2012;34(11):1524-1528.
- Veness MJ, Morgan GJ, Palme CE, Gebski V. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: combined treatment should be considered best practice. Laryngoscope. 2005;115(5):870-875.
- Veness MJ, Palme CE, Smith M, et al. Cutaneous head and neck squamous cell carcinoma metastatic to cervical lymph nodes (nonparotid): a better outcome with surgery and adjuvant radiotherapy. Laryngoscope. 2003;113(10):1827-1833.
- O’Brien CJ, McNeil EB, McMahon JD, et al. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck. 2002;24(5):417-422.
- Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27(10):843-850.
- Manyam BV, Garsa AA, Chin RI, et al. A multi-institutional comparison of outcomes of immunosuppressed and immunocompetent patients treated with surgery and radiation therapy for cutaneous squamous cell carcinoma of the head and neck. Cancer. Feb 07 2017. doi: 10.1002/cncr.30601.
- Buell JF, Hanaway MJ, Thomas M, Alloway RR, Woodle ES. Skin cancer following transplantation: the Israel Penn International Transplant Tumor Registry experience. Transplant Proc. 2005;37(2):962-963.
- Manyam BV, Gastman B, Zhang AY, et al. Inferior outcomes in immunosuppressed patients with high-risk cutaneous squamous cell carcinoma of the head and neck treated with surgery and radiation therapy. J Amer Acad Dermatol. 2015;73(2):221-227.
- Euvrard S, Morelon E, Rostaing L, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med.2012;367(4):329-339.
- Karia PS, Jambusaria-Pahlajani A, Harrington DP, et al. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014;32(4):327-334.
- Gluck I, Ibrahim M, Popovtzer A, et al. Skin cancer of the head and neck with perineural invasion: defining the clinical target volumes based on the pattern of failure. Int J Radiat Oncol Biol Phys. 2009;74(1):38-46.
- Becker LR, Aakhus AE, Reich HC, Lee PK. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017.153(4):321-322.
- Yom SS. Phase II Study of Radiation Therapy and Vismodegib for Advanced Head/Neck Basal Cell Carcinoma NCT01835626. 2017.
- Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945-1952.
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937-1944.
- Lewis CM, Glisson BS, Feng L, et al. A phase II study of gefitinib for aggressive cutaneous squamous cell carcinoma of the head and neck. Clin Cancer Res. 2012;18(5):1435-1446.
- Samstein RM, Ho AL, Lee NY, Barker CA. Locally advanced and unresectable cutaneous squamous cell carcinoma: outcomes of concurrent cetuximab and radiotherapy. J Skin Cancer. 2014;2014:284582.
- Chang AL, Kim J, Luciano R, et al. A case report of unresectable cutaneous squamous cell carcinoma responsive to pembrolizumab, a programmed cell death protein 1 inhibitor. JAMA Dermatol.2016;152(1):106-108.
- Mehra R, Seiwert TY, Mahipal A, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC): pooled analyses after long-term follow-up in KEYNOTE-012. J Clin Oncol. 2016;34(suppl; abstr 6012).
Citation
BV M, N J, SA K. A review of the role of external-beam radiation therapy in nonmelanomatous skin cancer. Appl Radiat Oncol. 2017;(2):6-10.
June 2, 2017