Aggressive Multimodality Therapy for Treatment of a Locally Advanced Radiation-Related Chest Wall Sarcoma
Images

Abstract
Radiation-induced soft-tissue sarcoma (STS) is a rare but serious long-term complication following radiation therapy. Management of these aggressive malignancies includes surgical resection with wide margins, as margin status has been consistently correlated with outcomes. Given the proximity to critical structures contained within the thoracic cavity, adequate margins are often difficult to achieve. Neoadjuvant therapy has become important to improve the probability of local control following surgical resection in locally advanced cases. Current clinical practice guidelines for STS recommend neoadjuvant therapy with radiation therapy, chemotherapy, or combination chemoradiation. While some studies have evaluated regional hyperthermia with chemotherapy or radiation, data regarding the efficacy of neoadjuvant thermochemoradiation are sparse. Specifically, treatment of chest wall STS with this multimodality regimen is not well documented. Here we present a patient who developed a 14-cm undifferentiated pleomorphic sarcoma of the chest wall 10 years after MammoSite (Cytyc/ Hologic) accelerated partial breast radiation. Due to the locally advanced nature of the primary tumor, neoadjuvant thermochemoradiation was delivered followed by an extensive chest wall resection with reconstruction.
Keywords: chest wall sarcoma, hyperthermia, chemoradiation, neoadjuvant radiation, radiation-induced sarcoma
Background
Breast-conserving therapy is a well-established treatment paradigm for early stage breast cancer, and accelerated partial breast irradiation (APBI) following lumpectomy has become a standard of care for many women. 1,2 MammoSite was the first FDA-approved device to deliver APBI, but due to the initial single lumen design, dose distribution could not be well optimized to limit chest wall and skin dose. 3 In a retrospective review, patients with a higher median chest wall dose were found to have a significantly higher risk of chest wall and rib pain following high dose rate brachytherapy, and newer multicatheter devices have been developed to permit improved dose optimization. 4 A late but serious complication of any radiation therapy includes the risk of secondary malignancy, a stochastic effect with a probability that is proportional to dose. 5,6 The incidence of radiation-induced sarcoma following breast radiation therapy is approximately 0.32% at 15 years compared with 0.23% in breast cancer patients not treated with radiation (P = 0.001). 7 Radiation-induced sarcomas are associated with poorer clinical outcomes when compared with sporadic cases, and local control can be challenging for locally advanced cases. 8 In one case-control series, the 5-year survival of patients with radiation-induced sarcoma was 32%, compared with 51% for sporadic sarcomas (P < 0.001). 9
Management of radiation-induced soft-tissue sarcoma (STS) largely depends on surgical resectability, with neoadjuvant chemotherapy and/or radiation therapy utilized to decrease the risk of local recurrence following surgery. For unresectable cases, definitive radiation therapy with or without chemotherapy can be used. 10 Hyperthermia is a known sensitizer that provides a synergistic effect when used in conjunction with radiation and chemotherapy. In this case study, we present a patient with a 14-cm radiation-in- duced undifferentiated pleomorphic sarcoma of the chest wall that was successfully resected following neoadjuvant thermochemoradiation with excellent outcomes.
Case Summary
This is a case of a 66-year-old woman who presented with a 1-year history of burning left upper quadrant abdominal and chest discomfort. Past medical history was significant for a T1bN0M0 invasive ductal carcinoma of the left breast, grade 1, ER 90%, PR 80%, and HER-2/neu negative 10 years prior. The patient was treated with standard breast-conserving therapy with partial mastectomy and sentinel lymph node biopsy followed by APBI and 5 years of anastrazole. APBI was delivered using the single lumen MammoSite applicator with iridium 192 high dose rate brachytherapy of 34 Gy in 10 fractions delivered twice per day over 5 consecutive days.
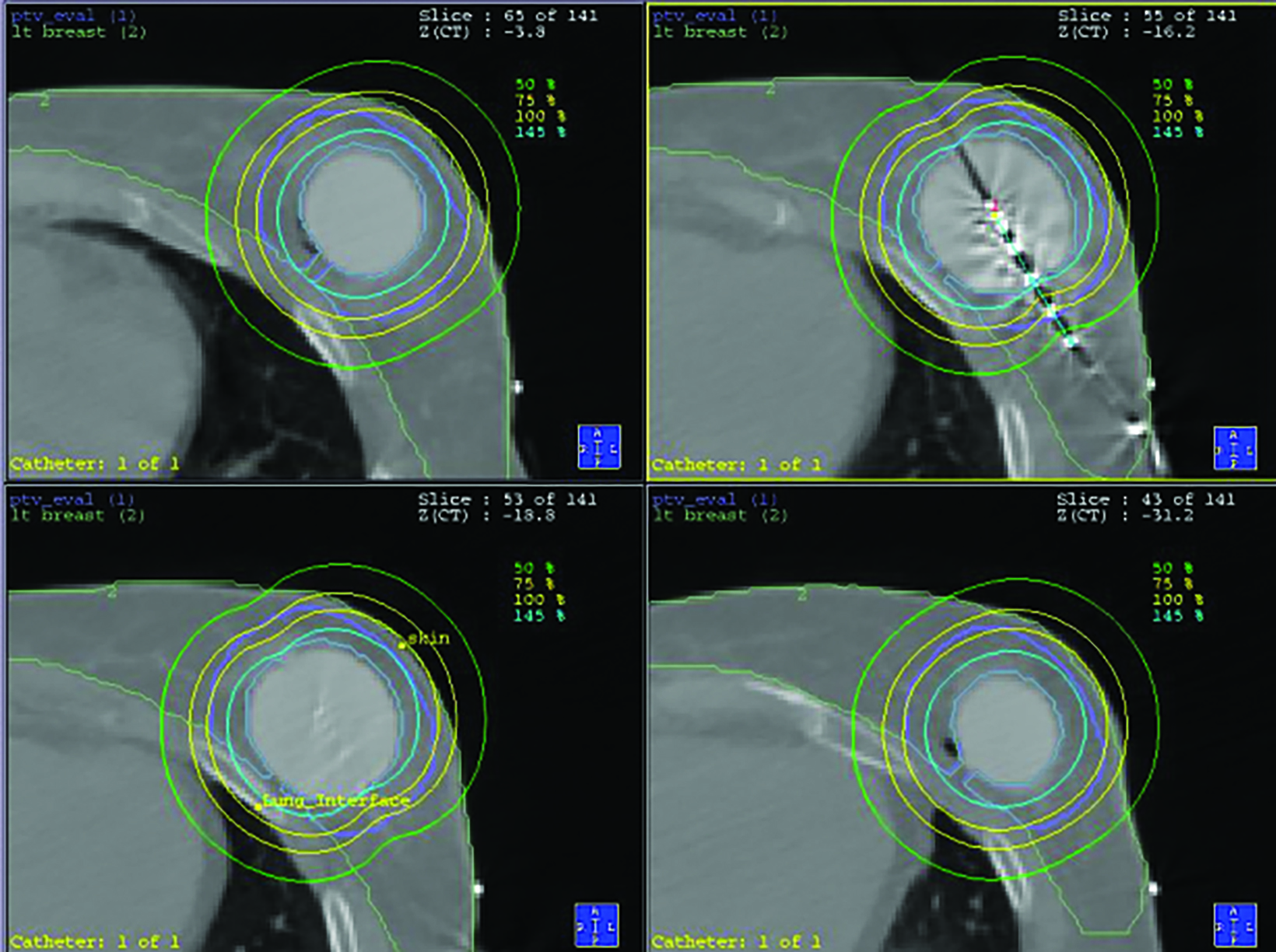
Due to the cavity size and location, a portion of the chest wall was within the 145% isodose line (Figure 1). The patient underwent routine annual mammogram screening for surveillance after treatment.
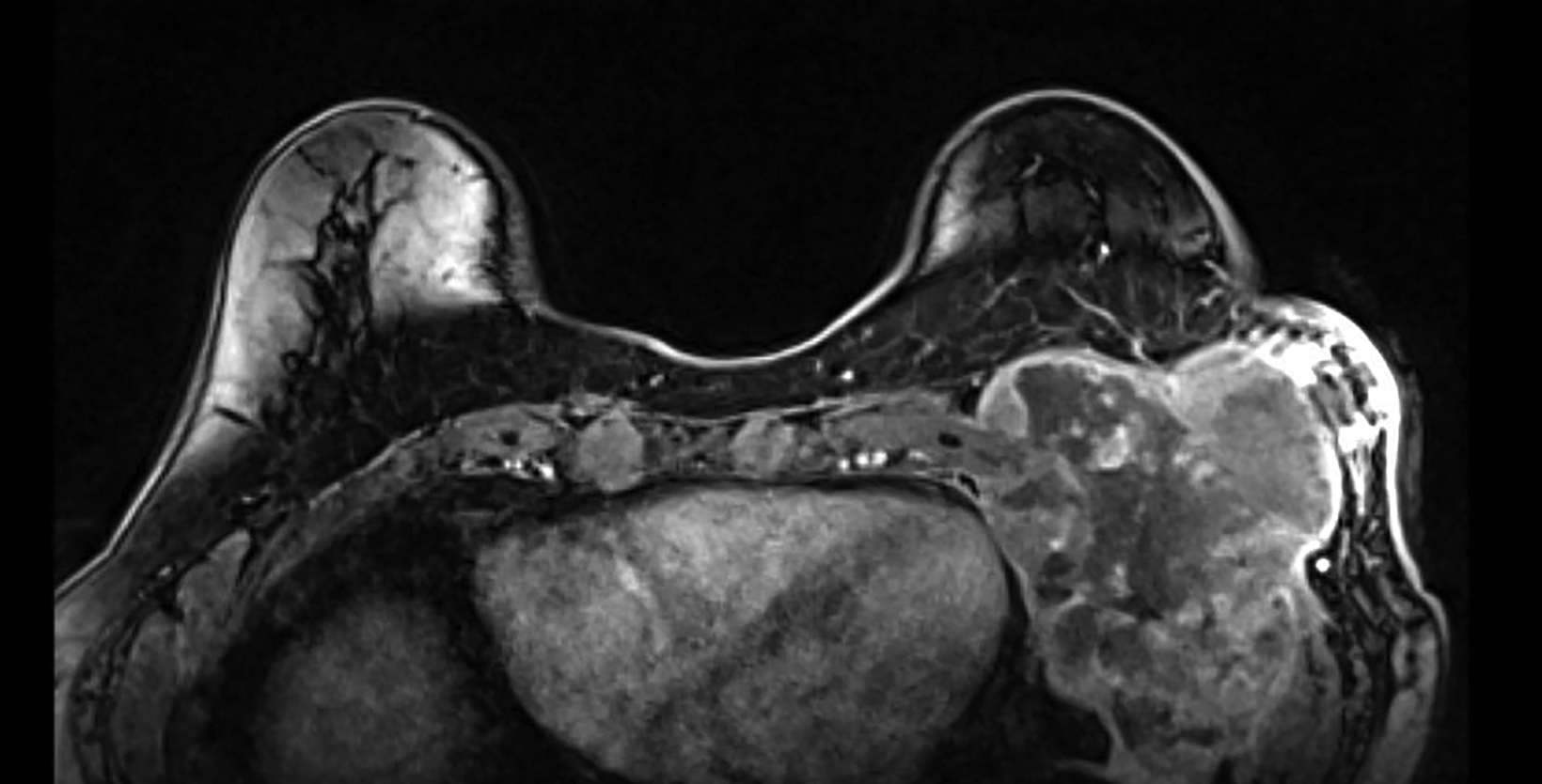
Workup of the patient’s upper abdominal/chest wall pain was initially limited as it was attributed to gastroesophageal reflux disease. The patient then noticed palpable changes at the site of prior lumpectomy, which were initially attributed to radiation fibrosis. On routine screening mammogram, an 8-cm mass of the left breast lower outer aspect was noted, which had not been seen on the mammogram from 16 months prior. Core needle biopsy of the left breast mass demonstrated a large cell malignant neoplasm, with immunostains favoring undifferentiated pleomorphic sarcoma, although a sarcomatoid carcinoma could not be excluded. MRI of the bilateral breasts with and without contrast demonstrated a 14 × 9 × 9-cm mass centered within the left chest wall, with invasion through the chest wall and suspected to be involving the pleura, pericardium, and left hemi-diaphragm (Figure 2). Positron emission tomography/CT (PET/CT) demonstrated the known primary mass with maximum standardized uptake value (SUV) of 25.5 and an internal mammary and prevascular lymph node with maximum SUV uptake of 2.5. Due to the architecture and low SUV uptake, the lymph nodes were favored to be reactive in nature. A follow-up MRI of the brachial plexus demonstrated no evidence of infiltration.
After multidisciplinary evaluation, we proceeded with neoadjuvant thermochemoradiation to improve likelihood of achieving local control following an anticipated close margin resection. Neoadjuvant radiation was planned to 50 Gy in 25 fractions delivered once daily with a 1-cm bolus applied to the chest wall for the first 13 fractions. Treatment was delivered with 3 coplanar volumetric-modulated arc therapy (VMAT) 10-MV arcs prescribed to the 97.3% planning target volume (PTV) mean (Figure 3). Active breathing control was utilized for motion management, and image guidance was provided with daily cone-beam computed tomography (CT) and surface-guided radiation therapy to monitor intrafraction motion. 11 Superficial hyperthermia was administered with a 915- MHz microwave applicator twice per week, separated by 72 hours, with the target temperature of 40 degrees Celsius for 60 minutes immediately prior to radiation. A large 20 × 20-cm applicator was used. Concurrent chemotherapy was administered with weekly gemcitabine at 500mg/m 2. The patient tolerated thermochemoradiation well with toxicity limited to grade 1 radiation dermatitis and fatigue.
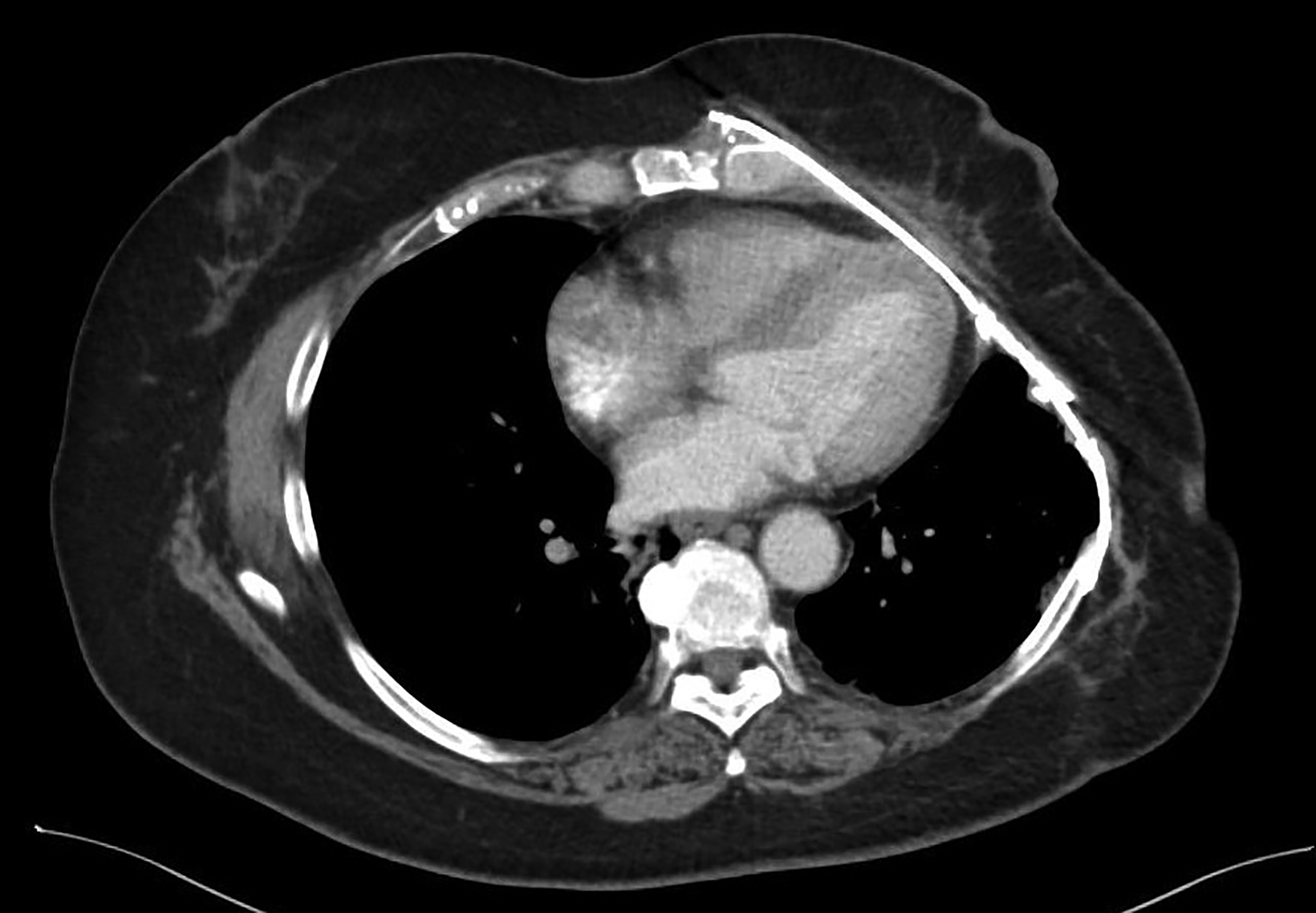
Five weeks following completion of thermochemoradiation, repeat CT of the chest with IV contrast demonstrated the known primary mass without a significant change in size. There was radiographic evidence of radiation pneumonitis in the left upper lung, for which the patient was asymptomatic, and a small pleural effusion. The imaging findings represented expected postradiation changes and there was no definitive evidence of distant metastatic disease, so the decision was made to proceed with surgical resection.
Seven weeks following the completion of neoadjuvant therapy, the patient underwent surgical resection of the large chest wall mass. Due to the locally advanced nature, resection of the chest wall – portions of ribs 3 to 8 – and wedge resection of the lung lingula were required. The postoperative defect measured 18 cm and was reconstructed with a 20 × 20 cm titanium mesh; a small residual defect was covered with prolene mesh (Figure 4). The titanium mesh has been increasingly used in our institution to provide a more rigid mechanical construct for large chest wall defects, which allows for improved ventilation mechanics. After the chest wall defect was closed, the plastic surgery team performed multisite reconstruction with latissimus dorsi myocutaneous flap, pectoralis minor muscle flap advancement, pectoralis major muscle flap advancement, and serratus muscle flap advancement. Pathological analysis was remarkable for ypT3 primary tumor with 70% necrosis, and final margins were negative with the closest margin at 0.3 cm (mediastinum). The patient tolerated the surgery well and was discharged home on postoperative day 5. There were no infectious, pulmonary, cardiac, or wound-healing complications. She underwent routine surveillance with CT imaging of the chest and physical examination every 3 months for the first 2 years and every 6 months to date. The patient is now 3.5 years post treatment, has resumed normal activities and has no evidence of disease. She was initially treated with gabapentin for mild chest wall pain, which was discontinued 1 year postoperatively. Currently, there is mild episodic nerve pain of the chest wall that lasts seconds and does not require any medical therapy.
Discussion
STS arising in a previously irradiated field often poses a therapeutic challenge, but neoadjuvant therapy can be critical in achieving an R0 resection for large tumors, providing the highest likelihood of local control. This patient’s tumor was initially deemed unresectable by multiple practitioners due to its extensive size. However, after detailed imaging, critical structures were determined to be tumor free and, although it was high-risk, a chest wall resection was deemed possible. Neoadjuvant therapy was crucial as the resection would be completed with limited margins and it also provided an opportunity to assess the biologic behavior of the tumor. It is important to note that the decision to proceed with aggressive therapy was made only after extensive discussion among the multidisciplinary tumor board. Sarcoma tumor board discussions can be particularly valuable, as comprehensive multidisciplinary treatment planning and care has been shown to be associated with improved 2-year, relapse-free survival in sarcoma patients (46.6% vs 51.7%, P < 0.001). 10 Additionally, treatment at higher volume centers has been associated with improved median survival (40 months vs 37 months, P = 0.002), highlighting the importance of multidisciplinary evaluation at tertiary care centers.
Gemcitabine was chosen as the concurrent chemotherapy agent as it is a well-known radiosensitizer, and phase I data from high-risk extremity and trunk STS demonstrated a major pathologic response (> 90% necrosis) in 47% of patients at a maximum tolerated dose of 700 mg/m 2. This study reported 5-year overall survival of 86%, but the maximum tolerated dose was associated with 24% grade 4 toxicity. 13 This study also did not have many trunk STSs, so there was additional concern for an increased risk of radiation pneumonitis that has been seen with gemcitabine and high-dose thoracic radiation in non-small cell lung cancer. 14,15 To provide the maximum benefit but limit risk of complications, which could delay or prevent surgery, gemcitabine was ultimately given at 500 mg/m 2 weekly, which was well tolerated. Previous literature suggests that gemcitabine acts as a potent radiosensitizer even at doses 1000 times lower than that normally achieved in plasma. 16
Due to the partially superficial nature of this chest wall tumor, the addition of moderate temperature hyperthermia was used as a complementary therapy. Hyperthermia results in enhanced perfusion improving oxygenation and, potentially, the effectiveness of chemoradiation. 17-20 Both hypoxia and radiation are known to induce expression of proteins, such as HIF-1α, which prevent activation of signaling cascades necessary to induce cellular apoptosis. Driving down expression of these proteins via oxygenation is thought to alleviate this blockade, increasing overall apoptosis from radiation-induced DNA damage. 20 In addition to enhanced perfusion and oxygenation, as intracellular temperature rises, tertiary and quaternary protein structure can be interrupted, resulting in denaturation and subsequent loss of function. Cytoskeletal elements, centrioles, and DNA repair proteins have been shown to be particularly sensitive to this form of damage. 21 Interruption of DNA repair mechanisms diminishes target cells’ ability to recover from both direct and indirect radiation-induced DNA damage. 22 This concept has been extended to DNA damage-based chemotherapeutic agents. 23 Specifically, hyperthermia has been shown to decrease cells’ ability to recover from gemcitabine-induced halted replication forks. 24 Our institution has a superficial microwave applicator that has a typical penetration of 3 cm and, although the entire tumor could not be completely heated, we felt that the possible benefit from heating the majority of the tumor with a low risk of toxicity justified its use.
The extensive chest wall resection that would be required to completely remove the tumor was the driving factor that led most providers to believe this tumor was not resectable. Any defect larger than 5 cm and involving multiple ribs must be carefully reconstructed to restore pulmonary function, protect the intrathoracic organs, and support soft-tissue reconstruction for wound closure. In this case, the resulting chest wall defect measured 18 cm and was reconstructed with titanium mesh and a complex multisite flap closure. Titanium mesh was chosen based on the biomechanical characteristics that create a stable and rigid anatomical chest wall contour while maintaining mechanical ventilation.
While these modalities have been studied in limited combination, the efficacy of thermochemoradiation prior to a large surgical resection requiring extensive reconstruction has not been well explored. In a phase III randomized study, the addition of regional hyperthermia to neoadjuvant systemic therapy for high-risk STS was shown to prolong median disease-free survival by 15.9 months and subsequently improve 5- and 10-year overall survival by 11% and 10%, respectively. 25 Hyperthermia with neoadjuvant chemoradiation was also found to double 3-year overall survival for patients with squamous cell carcinoma of the esophagus, and also resulted in a significantly higher rate of pathologic complete response (25% vs 5.9%, P < 0.05). 26 Hyperthermia can be particularly helpful for patients with unresectable disease, as the thermal enhancement ratio can result in a higher likelihood of local con- trol with definitive radiation. In the meta-analysis of radiation with hyperthermia for locally recurrent breast cancer, the addition of hyperthermia increased the likelihood of achieving a complete response by 22% without significant morbidity; hyperthermia was also associated with improved locoregional control in approximately two-thirds of patients receiving reirradiation. 27 In a more recent randomized control trial in cervical cancer, thermochemoradiation outperformed chemoradiation alone with higher 5-year overall survival rates (81.9% vs 72.3%, P < 0.05). 28 This case provides evidence that the successes seen with this aggressive multimodality approach have potential to extend to STS of the chest wall in patients with acceptable comorbidities.
Conclusion
This case report demonstrates the successful treatment of a patient with locally advanced radiation-induced chest wall sarcoma using neoadjuvant thermochemoradiation, surgical resection, and complex reconstruction with a titanium mesh implant and multisite flap closure. The aggressive treatment approach resulted in a microscopic complete resection and the patient remains disease free 3.5 years post treatment. While radiation-induced sarcomas present significant therapeutic challenges, it is important that otherwise fit patients without metastatic disease receive multidisciplinary evaluation at tertiary care centers, because with aggressive multimodality therapy they have potential for long-term survival.
References
- Vicini FA, Cecchini RS, White JR, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet. 2019;394:2155-2164. doi:10.1016/ S0140-6736(19)32514-0
- Whelan TJ, Julian JA, Berrang TS, et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet. 2019;394:2165-2172. doi:10.1016/ S0140-6736(19)32515-2
- Vicini FA, Douglas A, Todor D, Julian T, Lyden M. Dosimetric improvements in balloon-based brachytherapy using the contura R multi-lumen balloon (MLB) catheter to deliver accelerated partial breast irradiation. J Contemp Brachytherapy. 2010;2:1-8. doi:10.5114/jcb.2010.13716
- Brown S, Vicini F, Vanapalli JR, et al. Factors associated with chest wall toxicity after accelerated partial breast irradiation using high-dose-rate brachythera- py. Int J Radiat Oncol Biol Phys. 2012;83:801-805. doi:10.1016/j.ijrobp.2011.08.033
- Chang DS, Lasley FD, Das IJ, Mendonca MS, Dynlacht JR. Basic radiotherapy physics and biology. Basic Radiother Phys Biol. 2014;303-311. FF:10.1007/978-3-319-06841-1
- Santos AM, Marcu LG, Wong CM, Bezak E. Risk estimation of second primary cancers after breast radiotherapy. Acta Oncol. 2016;55:1331-1337. doi:10.1080/02 84186X.2016.1185150
- Yap J, Chuba PJ, Thomas R, et al. Sarcoma as a second malignancy after treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2002;52(5):1231-1237. doi:10.1016/s0360-3016(01)02799-7
- Dineen SP, Roland CL, Feig R, et al. Radiation-associated undifferentiated pleomorphic sarcoma is associated with worse clinical outcomes than sporadic lesions. Annals Surg Oncol. 2015;22:3913-3920. doi:10.1245/s10434-015-4453-z
- Bjerkehagen B, Småstuen M, Hall K, et al. Why do patients with radiation-induced sarcomas have a poor sarcoma-related survival? Br J Cancer. 2012;106:297- 306. doi:10.1038/bjc.2011.559
- Mehren MV, Randall RL, Benjamin RS, et al. Soft Tissue Sarcoma, Version 2.2018: Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2018;16:536-563. doi:10.6004/jnccn.2018.0025
- Eldredge-Hindy H, Lockamy V, Crawford A, et al. Active Breathing Coordinator reduces radiation dose to the heart and preserves local control in patients with left breast cancer: report of a prospective trial. Pract Radiat Oncol. 2015;5:4-10. doi:10.1016/j.prro.2014.06.004.Active
- Blay J-Y, Soibinet P, Penel N, et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann Oncol. 2017;28:2852-2859. doi:10.1093/annonc/mdx484
- Tseng WW, Zhou S, To CA, et al. Phase 1 adaptive dose-finding study of neoadjuvant gemcitabine combined with radiation therapy for patients with high- risk extremity and trunk soft tissue sarcoma. 2015;121(20):3659-3667. doi:10.1002/cncr.29544
- Arrieta O, Gallardo-Rincon D, Villarreal-Garza C, et al. High frequency of radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent radiotherapy and gemcitabine after induction with gemcitabine and carboplatin. J Thorac Oncol. 2009;4:845-852. doi:10.1097/ JTO.0b013e3181a97e17
- Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457-2463. doi:10.1200/JCO.2007.14.7371
- Shewach DS, Lawrence TS. Gemcitabine and radiosensitization in human tumor cells. Investig New Drugs. 1996;14:257-263. doi:10.1007/BF00194528
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res. 1984;44(10 Suppl):4721s-4730s.
- Brizel DM, Scully SP, Harrelson JM, et al. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res. 1996;56:5347-5350.
- Sun X, Xing L, Clifton Ling C, Li GC. The effect of mild temperature hyperthermia on tumour hypoxia and blood perfusion: relevance for radiotherapy, vascular targeting and imaging. Int J Hyperth. 2010;26:224-231. doi:10.3109/02656730903479855
- Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429-441. doi:10.1016/S1535-6108(04)00115-1
- Vidair CA, Doxsey SJ, Dewey WC. Heat shock alters centrosome organization leading to mitotic dysfunction and cell death. J Cell Physiol. 1993;54:443-455. doi:10.1002/jcp.1041540302
- Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol.2001;77:399-408. doi:10.1080/09553000010024687
- Mantso T, Goussetis G, Franco R, Botaitis S, Pappa A, Panayiotidis M. Effects of hyperthermia as a mitigation strategy in DNA damage-based cancer therapies. Semin Cancer Biol. 2016;37-38:96-105. doi:10.1016/j.semcancer.2016.03.004
- Raoof M, Zhu C, Cisneros BT, et al. Hyperthermia inhibits recombination repair of gemcitabine-stalled replication forks. J Natl Cancer Inst. 2014;106(8). doi10.1093/jnci/dju183
- Issels R, Linder LH, Verweij J, et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma. 2018;4:483-492. doi:10.1001/jamaoncol.2017.4996
- Kitamura K, Kuwano M, Watanabe M, et al. Prospective randomized study of hyperthermia combined with chemoradiotherapy for esophageal carcinoma. J Surg Oncol. 1995;60;55-58. doi:10.1002/jso.2930600111
- Datta NR, Puric E, Klingbiel D, Gomez S, Bodis S. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94:1073-1087. doi:10.1016/j.ijrobp.2015.12.361
- Wang Y, Hong W, Che S, et al. Outcomes for hyperthermia combined with concurrent radiochemotherapy for patients with cervical cancer. Int J Radiat Oncol Biol Phys. 2020;107(3):499-511. doi:10.1016/j.ijrobp.2020.03.006
Citation
N F, DP R, G S, D S, CS S, N M, JG S, SR C. Aggressive Multimodality Therapy for Treatment of a Locally Advanced Radiation-Related Chest Wall Sarcoma. Appl Radiat Oncol. 2022;(3):39-44.
October 14, 2022