Artificial Intelligence in Radiation Therapy: Adaptive Applications and Beyond
Artificial intelligence (AI) has emerged as a transformative tool in health care, its use expanding in various areas, including radiation therapy. From autosegmentation to treatment planning to adaptive radiation therapy (ART), AI and deep-learning methods are beginning to impact a wide range of applications. AI supports radiation oncologists in their effort to localize the treatment of tumors while minimizing the effects of radiation on healthy tissue. Once consistency and accuracy are achieved in the clinical setting, the tool will help clinicians provide this benefit with ART to recalculate treatment plans based on daily—or even moment-to-moment—changes in patient anatomy, particularly in areas prone to variation like the abdomen, thorax, and prostate. AI tools aren’t limited to adaptive treatment in specific disease sites, however, as they’re also applied to areas such as the head and neck in response to treatment changes.
Clinical adoption of AI in radiation therapy—particularly as it relates to ART—has been accelerating but slowly so. While it’s certain that AI can significantly improve accuracy, personalization, and efficiency of RT delivery, challenges remain regarding availability of data sets, training, validation, and overall efficacy in clinical application. As the industry continues to explore how AI can and will transform clinical practice, the concept of continuous adaptation in radiation therapy garners excitement and anticipation among stakeholders in the specialty.
Speed Creating Opportunity
Traditional processes for establishing the radiation treatment plan in a multidisciplinary environment remain complex, labor-intensive and, for these reasons, relatively lengthy. It typically takes 1 to 2 weeks to evaluate the patient, take scans, delineate the target and organs at risk, and develop a robust treatment plan that will attempt to satisfy the patient’s daily variable presentation, explains Jan Jakob Sonke, PhD, group leader in adaptive radiation therapy at the Netherlands Cancer Institute and professor by special appointment at the University of Amsterdam, who notes this robustness comes with a cost: radiation to some healthy tissue.
“ART aims to adapt the treatment plan to the anatomical and/or biological changes in the patient over the course of radiation therapy. To that end, the patient is re-imaged in the treatment room, the images are quantitatively analyzed to quantify changes, and the treatment plan is subsequently re-optimized to account for these changes. Traditionally, ART is labor intensive so [it is] applied restrictively,” Dr. Sonke explains, noting that if the ART process could be accelerated via AI to occur in a mere few minutes, the treatment team could perform a more accurate delivery right on the table. “AI has the potential to improve all components of the adaptive feedback loop in terms of quality and efficiency and maximize the impact of ART.”
AI may play a role in image reconstruction to reduce acquisition time, reduce imaging dose, and improve in-room image quality, Dr. Sonke adds. Similarly, AI may automate recontouring of the daily images and improve deformable image registration to quantify anatomical changes in 3-D. The tools may also contribute to treatment plan adaptation.
Elekta’s MR-Linac, Unity, is a dedicated adaptive platform for radiation therapy. photo/courtesy Elekta
Yielding increased speed of critical RT functions, opportunities enabled by AI will have a lasting impact on clinician workload, says Rui Lopes, MBA, director of new technology assessment at Elekta, the Stockholm-based manufacturer of the Elekta Unity MR-linac (pictured above). “By automating or enhancing time-intensive tasks that have been traditional bottlenecks in the process—like organ segmentation, speeding up and improving image acquisitions, and automated planning—AI is allowing clinicians to focus more of their attention on the individual patient and on personalizing treatment, including treatment adaptation when necessary,” Lopes says, noting that as online adaptation becomes quicker, ART becomes a more viable option.
Fredrik Löfman, PhD, director of machine learning at RaySearch Laboratories, based in Stockholm, says that AI harnesses benefits in automating specific, recurrent RT tasks, such as segmentation of image sets and treatment plan generation. Automation through AI presents “a very qualified first guess” of these functions that must be reviewed and possibly fine-tuned by the user. Still, it can save a lot of valuable time, he says.
In current applications of RT clinical practice, AI is applied with a human in the loop to check the computer’s assessment prior to deployment. “AI in ART is pretty new ground,” Dr. Löfman explains. “Even just with AI in radiation therapy, how do you monitor model performance over time? How should you validate? How do you ensure the physicians or treatment planners are active even though there’s automation?” he asks. “Then with adaptive, things get harder because you have less time. The patient is on the couch and every second counts. What’s the process to review and approve them before treatment is started, and how much time should that process take?” More research is needed to address these unknowns, he says.
Data Highlights and Challenges
While there are many papers on AI applications in RT, only a limited number of trials have tested clinical implementation. A recent study in Nature Medicine compared physician evaluations of radiation treatments generated by an AI machine-learning (ML) algorithm to conventional radiation treatments.1 The researchers found that in the majority of 100 patients studied, treatments generated using ML were deemed clinically acceptable for patient treatments by physicians, and the process was 60% faster. In an ART-focused study, researchers demonstrated an online solution’s feasibility for various pelvic sites, with clinically acceptable AI-segmentation and auto-planning enabling adaptation within reasonable timeslots.2
Some working groups, consortiums and collaborative ventures are also underway. The Netherlands Cancer Institute, the University of Amsterdam, and Elekta are engaged in a Partnership for Online Personalized AI-driven Adaptive Radiation Therapy (POP-AART), a lab where researchers develop novel AI strategies for improving images on which radiation treatments are based, predicting changes of tumors over time and incorporating them in automatic treatment planning and adaptation.3 While adapting the plan at every treatment session has been challenging due to computational and workflow complexities, AI has the potential to make this available to many patients in the near future, says Dr. Sonke, one of the lab directors.
In terms of big data, the researchers are pioneering the use of novel ML/deep-learning (DL) approaches to address RT challenges including personalization, speed, and precision. In general, high-quality big data from multiple institutions are highly expected to further improve AI algorithms, says X. Sharon Qi, PhD, DABR, associate professor of medical physics at the University of California Los Angeles. “The AI tools, so far, were generally developed and evaluated by a single (or a couple of) institution(s) using their own training and testing datasets. The performance of the tools has yet to be further evaluated using larger independent datasets across multiple institutions,” she says, noting that interobserver or intraobserver variability are widely known, which creates additional challenges relating to ground truth data for algorithm development.
Dr. Sonke continues: “An important challenge of AI in ART is generalizability. AI algorithms are typically trained on the available limited data. The question is [whether] the algorithm still performs well in new data that the algorithm hasn’t seen. These data may be from different institutes, scanners, and clinical protocols. Efforts to generate large, multi-institutional databases may help to alleviate this challenge.”
Dr. Löfman adds that amassing very curated data sets from clinical data that must be aligned and organized is a primary challenge for ART. “It’s really a data problem; that’s no surprise,” he says. He also notes that explainable AI will increase to bring more transparency about whether the algorithm works—or not—and why it behaves as it does.
Future Directions
Just as the concept of self-driving cars has not translated to drivers “sleeping at the wheel,” AI in RT will need continuous oversight and monitoring as its usage increases. Dr. Qi notes that the radiation oncology community needs to develop guidance and recommendations for clinical commissioning, validation, implementation, and maintenance of AI-based tools. In addition, it’s crucial to set up robust quality assurance (QA) programs.
“We need to implement AI tools with caution, setting up our clinical QA process effectively to take advantage of AI-based tools,” she says. Taking auto-segmentation as an example, AI can speed up the contouring process from hours to minutes. Dr. Qi adds that the overall time spent on AI segmentation should account for the extra time spent on human review and correction, “an area that still needs to get worked out.”
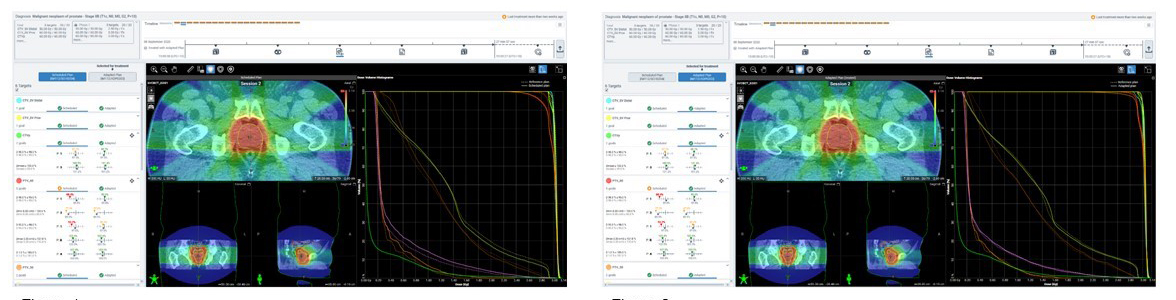
For one representative fraction, the left image shows the scheduled plan and the right shows the optimized adapted
plan for a patient with prostate cancer. In this instance, the adaptive plan was selected because it outperformed the scheduled plan on meeting PTV60 goals. Image courtesy of Icon Cancer Centre at Sydney Adventist Hospital
“The possibilities of AI are in their infancy,” says Sasa Mutic, senior VP of Radiation Treatment Solutions at Varian, which offers Ethos (treatment plan example pictured above). “Five years ago, most of us did not predict the use of AI in radiation therapy. We also wouldn’t have believed that online ART with a completely new plan could be accomplished within a typical 15-minute time slot, but our customers systematically do that today with Ethos. Based on these results, we are emboldened to invest even more in the use of AI in radiation therapy and ART, both online and offline.”
With numerous clinicians impacted by automation in the RT workflow and its associated developments, it’s critical to utilize a team approach to AI implementation. “Adopting novel technology in RT like AI should be a multidisciplinary effort. Gathering a team of motivated experts including radiation oncologists, medical physicists, radiation technologists, computer scientists, and IT will help to maximize AI’s clinical benefit,” Dr. Sonke says, noting the importance of a collaborative investment at the earliest stages of innovation to define goals, expectations, and discuss impact on workflow.
“Ideally we unburden clinicians from routine tasks so that they can focus on the patient,” Lopes concludes. “The appetite for simplifying and streamlining RT workflows while maintaining safety and efficacy is tremendous.”
References
- McIntosh C, Conroy L, Tjong MC, et al.Clinical integration of machine learning for curative-intent radiation treatment of patients with prostate cancer. Nat Med. 2021;27:999-1005. https://doi.org/10.1038/s41591-021-01359-w
- Sibolt P, Andersson LM, Calmels L, et al. Clinical implementation of artificial intelligence-driven cone-beam computed tomography-guided online adaptive radiotherapy in the pelvic region, Phys Imag Rad Oncol. 2021;17:1-7. https://doi.org/10.1016/j.phro.2020.12.004
- University of Amsterdam. AI can help improve precision radiotherapy. Accessed February 25, 2022. https://www.uva.nl/en/content/news/press-releases/2021/07/ai-can-help-improve-precision-radiotherapy.html?cb