Barrigel Rectal Spacer Gets FDA Clearance
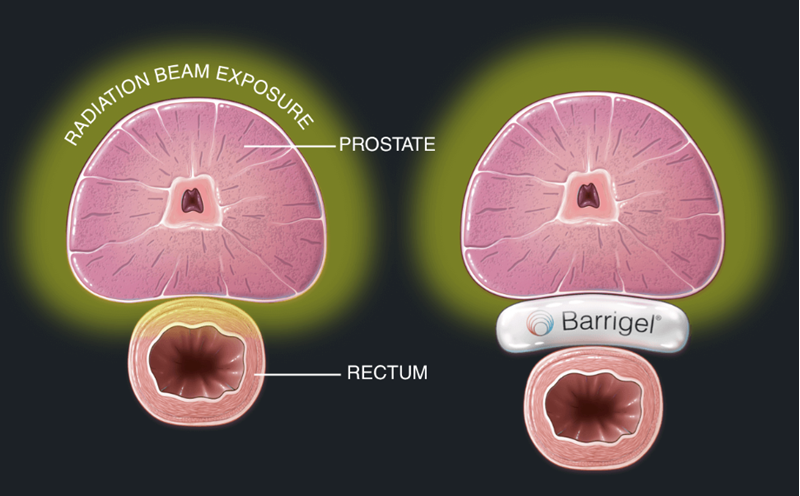 Palette Life Sciences announced US FDA clearance of Barrigel, the first and only hyaluronic acid rectal spacer that separates the prostate from the rectum to protect it during radiation therapy treatment for prostate cancer. The Barrigel clearance is based on data from the FDA-reviewed randomized controlled trial using hypofractionated radiotherapy for treating prostate cancer with a rectal spacer. Barrigel is indicated for prostate cancer patients with T1-T3b disease.
Palette Life Sciences announced US FDA clearance of Barrigel, the first and only hyaluronic acid rectal spacer that separates the prostate from the rectum to protect it during radiation therapy treatment for prostate cancer. The Barrigel clearance is based on data from the FDA-reviewed randomized controlled trial using hypofractionated radiotherapy for treating prostate cancer with a rectal spacer. Barrigel is indicated for prostate cancer patients with T1-T3b disease.
“We are extremely proud of today’s US FDA clearance of Barrigel and we look forward to working with urologists and radiation oncologists to help improve outcomes for the approximately 250,000 men in the US diagnosed with prostate cancer each year, and 1.4 million men diagnosed globally,” said Per G. Langö, Chief Executive Officer and Board Director of Palette Life Sciences. “Data show that personalized medicine such as precision radiation therapy offers patients the greatest chance of survival and using Barrigel can decrease potential damage to healthy tissue during this treatment.”
“The Barrigel Prostate Trial found that 98.5% of men who were treated with Barrigel met the primary endpoint of achieving at least a 25% reduction in radiation to the rectum. Patients who met the primary endpoint averaged an 85% reduction in radiation to the rectum, and Barrigel is proven superior in the reduction of acute and long-term Grade 2+ GI toxicity at 3 and 6 months compared to control.” said Dr Martin King, Assistant Professor of Radiation Oncology at Harvard Medical School, Dana-Farber Cancer Institute in Boston and Medical Director of the Barrigel Prostate Trial.
“Radiation therapy is a cornerstone of prostate cancer treatment, but radiation exposure to surrounding healthy tissue, including the rectum, can profoundly impact the quality of life of these cancer survivors,” said Dr Peter F. Orio III, Associate Professor of Radiation Oncology at Harvard Medical School. “There is a trend to use hypofractionated radiotherapy, which uses a shorter course of radiation to allow patients to complete their treatment in four to six weeks compared to conventionally fractionated radiotherapy, which takes almost nine weeks to complete. Quicker courses of radiation are more convenient to the patient and have been proven to provide equal cancer control rates, but can come at the cost of causing additional toxicity to patients, especially to the rectum, as a greater amount of radiation must be delivered each day in their course of therapy. Barrigel, injected between the prostate and the rectum, supports higher radiation doses while lowering the risk of toxicity to the rectum, resulting in less treatment-related side effects for these men.”