CHHiP vs PROFIT for Localized Prostate Cancer: A Retrospective Dosimetric Comparison of Organs at Risk
Affiliations
- 1 Department of Radiation Oncology, American University of Beirut Medical Center, Beirut, Lebanon
- 2 Department of Radiation Oncology, Nabatieh Governmental Hospital, Nabatieh, Lebanon
Objectives
Moderate hypofractionation for localized prostate cancer has become a standard of care in many radiation therapy centers worldwide. Several fractionation and planning protocols exist, with CHHiP and PROFIT (60 Gy in 20 fractions) being 2 of the most commonly used. We retrospectively compared the doses received by organs at risk (OARs) using these 2 protocols.
Materials and Methods
We retrospectively reviewed the charts of 25 randomly selected de-identified patients treated with intensity-modulated radiation therapy (IMRT) for prostate cancer in a single tertiary care center. For each patient, we generated 2 sets of contours for target volumes and OARs in accordance with both CHHiP and PROFIT protocols. A total of 50 IMRT plans, using Prowess Panther software version 5.10, were generated and achieved the respective planning targets and normal tissue constraints. The related-samples Wilcoxon signed-rank test was used to compare the mean dose, V60, V50, and V40 of each of the bladder, rectum, and penile bulb.
Results
Patients had a mean age of 73 years, average prostate-specific antigen level of 9.8 ng/mL, mostly a Gleason score of 7, and a clinical stage that ranged from T1c to T2c. In the CHHiP plans, the rectum averaged a significantly lower V60 (0.5% vs 4.5%, P < .001) and V50 (13.1% vs 15.7%, P = .026) than with PROFIT. Similarly, the bladder in CHHiP averaged a significantly lower V60 (1.9% vs 7.7%, P < .001) and V50 (13.2% vs 15.5%, P = .035). The penile bulb received a lower mean dose (21.9 Gy vs 30.5 Gy, P < .001), V50 (5.6% vs 14.4%, P = .037), and V40 (11.4% vs 35.2%, P < .001) on average in the CHHiP plans as well.
Conclusion
In our dosimetric comparison, CHHiP spared the OARs to a greater degree than PROFIT. While contouring and planning using the CHHiP protocol are usually more demanding, we expect that greater sparing of OARs will minimize clinical toxicity in patients with prostate cancer receiving moderately hypofractionated radiation therapy.
Introduction
Prostate cancer is the second most common cancer in men, with more than 1.4 million estimated new cases in the year 2020 alone.1 Radiation therapy is a standard treatment option for patients with localized disease in all risk categories, and it can be used as monotherapy or in combination with systemic therapies.2 The response of cells to radiation is depicted in a linear-quadratic model incorporating a tissue-specific α/β ratio, which informs how sensitive the tissue is to fractionation. Prostate cancer has a low α/β value, suggesting that delivering higher radiation doses per fraction (ie, hypofractionation) may provide a therapeutic advantage.3 Several randomized trials, including CHHiP and PROFIT, compared hypo-fractionated radiation regimens (>2 Gy per fraction) with standard fractionation (1.8-2 Gy per fraction), showing noninferiority in clinical outcomes and acceptable toxicity profiles.4 - 8 These favorable results have made moderate hypofractionation (2.4-3.4 Gy per fraction) the preferred approach at many institutions worldwide in the treatment of low- and intermediate-risk prostate cancer9 as it allows the reduction of treatment time by half.
Two commonly used radiation regimens are those applied in CHHiP and PROFIT, both employing 60 Gy in 20 fractions. While the dose and fractionation are similar in the 2 regimens, there are large differences in the target and normal tissues delineation between them. Notably, the PROFIT protocol prescribes the total dose to a single target volume with volumetric expansions, whereas CHHiP prescribes 3 different doses to 3 target volumes using a simultaneous integrated boost technique, in such a way that the prostate receives 60 Gy in both protocols.6, 8
While both protocols had acceptable toxicity compared with standard fractionated radiation therapy,6, 8 they have not been tested head-to-head to compare their toxicity profiles. Therefore, aside from single institutional preferences, it is unknown whether one protocol can offer an advantage in decreasing normal tissue toxicity over the other. In an attempt to fill this knowledge gap, we retrospectively compared the doses received by organs at risk (OARs) using these 2 protocols in a homogeneous patient population treated at our institution from January 2017 to January 2020.
Materials and Methods
Patient Selection and Simulation
We retrospectively reviewed the charts of 25 randomly selected patients treated with intensity-modulated radiation therapy (IMRT) for prostate cancer in a single tertiary care center between January 2017 and January 2020. Only adult patients who received definitive radiation therapy at our institution for localized prostate cancer were included. Patients who underwent radical prostatectomy or who had metastatic disease were excluded. The study was approved by our institutional review board.
As per institutional practice, all patients were originally planned and treated with the CHHiP protocol. Each of these patients had a planning CT simulation with a full bladder and an empty rectum.
Contouring and Treatment Planning
For each patient, we generated 2 sets of contours for target volumes and OARs in accordance with both CHHiP and PROFIT protocols. The CHHiP protocol stratifies patients into either low- or moderate-/high-risk groups based on the T stage and the risk of seminal vesicle invasion (SVI) as per the Roach formula: PSA + (10 × [Gleason score - 6]).10 If a patient has a T stage of T2c or T3a or an SVI risk > 15%, then he is deemed to be at moderate/high risk for SVI. The PROFIT protocol also stratifies patients based on the risk of SVI, but it uses the Partin tables instead, with the cutoff being 15% as well.11, 12
The CHHiP protocol requires the contouring of 3 different target volumes planned to different doses using simultaneous integrated boost technique, whereas the PROFIT protocol requires only a single target volume. Table 1 compares both regimens in terms of target delineation.
Comparison of CHHiP and PROFIT Protocols in Terms of Target Delineation
| CHHiP | PROFIT | |
|---|---|---|
| Patient risk stratification | Yes | Yes |
| Estimation of SVI risk | Roach formula | Partin table |
| Number of target volumes | 3 | 1 |
| Clinical target volumes | ||
| CTV1 | CTV | |
| If low risk of SVI: prostate gland + base of SV + 5 mm If moderate/high risk of SVI: prostate gland + SV + 5 mm |
If risk of SVI < 15%: prostate gland only If risk of SVI > 15%: prostate gland + proximal 1 cm of SV |
|
| PTV1 = CTV1 + 5 mm | PTV = CTV + 10 mm (7 mm posteriorly) | |
| 48.0 Gy | 60.0 Gy | |
| CTV2 | ||
| If low risk of SVI: prostate gland + 5 mm If moderate/high risk of SVI: prostate gland ± base of SV * + 5 mm |
||
| PTV2 = CTV2 + 5 mm (0 mm posteriorly, or 5 mm if rectum is moderate-large in size) | ||
| 57.6 Gy | ||
| CTV3 | ||
| Prostate gland only | ||
| PTV3 = CTV3 + 5 mm (0 mm posteriorly) | ||
| 60.0 Gy |
Abbreviations: CTV, clinical target volume; PTV, planning target volume; SVI, seminal vesicle invasion.
*Include the base of the seminal vesicles in CTV2 if seminal vesicle invasion is evident on MRI (ie, if the tumor has a clinical stage of T3b).
The protocols also differ in contouring of the OARs, especially the bladder and the rectum. CHHiP contours them as solid organs, while PROFIT only contours the bladder and rectal walls. The penile bulb is contoured in the CHHiP protocol but not in PROFIT. Table 2 compares both regimens in terms of OAR delineation.
Comparison of CHHiP and PROFIT Protocols in Terms of Organs at Risk (OAR) Delineation, Prescription Aims, and Normal Tissue Dose Constraints13
| OAR | CHHiP | PROFIT |
|---|---|---|
| OAR delineation | ||
| Bladder | Solid organ, from base to dome | Bladder wall (3 mm ring), for 18 mm below and above the contoured CTV |
| Rectum | Solid organ, from anus to recto-sigmoid junction | Rectal wall (3 mm ring), for 18 mm below and above the contoured CTV |
| Penile bulb | Contoured | Not contoured |
| Femoral head and neck | Contoured | Contoured |
| Prescription aims and dose constraints | ||
| CTV | D99 ≥ 60 Gy | |
| PTV | D99 ≥ 57 Gy | D99 ≥ 57 Gy |
| D1cc ≤ 63 Gy | D1cc ≤ 63 Gy | |
| Bladder | V60 ≤ 5% V48 ≤ 25% V40 ≤ 50% |
V46 ≤ 30% V37 ≤ 50% |
| Rectum | V57 ≤ 15% V40 ≤ 60% |
V46 ≤ 30% V37 ≤ 50% |
| Penile bulb * | V40 ≤ 50% | Not applicable |
| Femoral head | V40 ≤ 50% | V43 ≤ 5% |
Abbreviations: CTV, clinical target volume; PTV, planning target volume.
*The values for the penile bulb are nonmandatory constraints as per CHHiP and are for clinician guidance only.
Using Panther software (Prowess Inc.) version 5.10, IMRT plans were generated (total 50 plans). Assessment and approval of the plans followed the guidelines of the 2 protocols, relying on their respective planning targets and normal tissue constraints, as summarized in Table 2 .
Plan Comparison
In treatment planning and approval, the CHHiP protocol contours the whole bladder and rectum, whereas the PROFIT protocol only considers the bladder and rectal walls as elaborated above. For the purpose of comparing the doses received by the OARs, namely the bladder, rectum, and penile bulb, whole organ contours were considered. Using Panther software version 5.10, we extracted the mean doses to the OARs as well as the percentage of each volume receiving at least 40 Gy, 50 Gy, or 60 Gy (V40, V50, and V60, respectively). Even though the femoral heads and necks were contoured, dose volume data were not extracted for this OAR as their location is not in the vicinity of the prostate and the comparison was deemed not to be relevant herein.
Statistical Analysis
For the statistical analysis, we used IBM SPSS Statistics for Windows, version 28 (IBM Corp.). Given that we have 25 patients only, we relied on nonparametric tests for comparison, namely the related-samples Wilcoxon signed-rank test, with the significance level being 0.05.
Results
Patient Characteristics
A total of 25 patient charts were reviewed, and a total of 50 patient plans were generated. Patients had a mean age of 73 years (range, 54-79 years), an average prostate-specific antigen (PSA) level of 9.8 ng/mL (range, 2-25 ng/mL), mostly a Gleason score of 7 (range, 6-7), and a clinical stage that ranged from T1c to T2c, with most patients having a stage of either T2a or T2c. As per the CHHiP protocol, the majority of patients were labeled as having a high risk of SVI (80%). On the other hand, as per the PROFIT protocol, only 16% were considered to have a high risk of SVI. Table 3 summarizes the patient characteristics and their respective risk of SVI in either CHHiP or PROFIT. The exact risk percentages of SVI can be found in the supplementary information found in the online version of this article (Table S1).
Characteristics of the Patients and Their Respective Risk of Seminal Vesicle Invasion (SVI) as per CHHiP and PROFIT
| AGE (YEARS) | PSA (ng/mL) | GLEASON SCORE | CLINICAL STAGE | CHHiP SVI RISK |
PROFIT SVI RISK |
|
|---|---|---|---|---|---|---|
| 1 | 66 | 10.5 | 7 (4 + 3) | T1c | High | High |
| 2 | 75 | 8.5 | 7 (4 + 3) | T2a | High | Low |
| 3 | 68 | 13.0 | 7 (3 + 4) | T2a | High | Low |
| 4 | 54 | 7.2 | 6 (3 + 3) | T1c | Low | Low |
| 5 | 76 | 15.0 | 7 (3 + 4) | T2a | High | Low |
| 6 | 78 | 15.0 | 7 (4 + 3) | T2c | High | High |
| 7 | 79 | 11.0 | 6 (3 + 3) | T2a | Low | Low |
| 8 | 74 | 12.0 | 6 (3 + 3) | T2b | Low | Low |
| 9 | 78 | 14.7 | 7 (3 + 4) | T2b | High | Low |
| 10 | 70 | 25.0 | 7 (4 + 3) | T1c | High | High |
| 11 | 72 | 6.2 | 7 (4 + 3) | T1c | High | Low |
| 12 | 70 | 6.7 | 7 (4 + 3) | T2a | High | Low |
| 13 | 77 | 8.0 | 6 (3 + 3) | T2a | Low | Low |
| 14 | 78 | 21.0 | 7 (4 + 3) | T2c | High | High |
| 15 | 73 | 6.2 | 7 (3 + 4) | T2c | High | Low |
| 16 | 74 | 10.8 | 6 (3 + 3) | T2c | High | Low |
| 17 | 76 | 11.6 | 6 (3 + 3) | T2c | High | Low |
| 18 | 79 | 6.5 | 7 (3 + 4) | T2a | High | Low |
| 19 | 78 | 5.2 | 7 (3 + 4) | T2a | High | Low |
| 20 | 75 | 5.3 | 7 (4 + 3) | T2c | High | Low |
| 21 | 76 | 9.8 | 7 (4 + 3) | T2a | High | Low |
| 22 | 78 | 4.3 | 7 (3 + 4) | T2c | High | Low |
| 23 | 78 | 3.8 | 7 (4 + 3) | T2b | Low | Low |
| 24 | 73 | 2.0 | 7 (4 + 3) | T2c | High | Low |
| 25 | 56 | 5.7 | 7 (4 + 3) | T2c | High | Low |
Abbreviation: PSA, prostate-specific antigen.
CHHiP uses the Roach formula, whereas PROFIT uses the Partin tables.
Target Coverage
All plans followed the respective protocols in terms of target coverage and satisfied the prescription aims, which are depicted in Table 2 . We did not compare target coverage between either protocol as our objective involves OAR sparing only.
Doses to Organs at Risk
In the CHHiP protocol, the bladder and the rectum were less exposed to higher radiation doses, manifesting as lower V60 and V50 on average than in the PROFIT protocol. The differences were statistically significant ( P value < .05). The mean dose and V40 were similar.
As for the penile bulb, the mean dose was significantly less with CHHiP than with PROFIT (21.9 vs 30.5 Gy, respectively, P value < .001). V50 and V40 were also significantly lower with CHHiP (5.6% vs 14.4% and 11.4% vs 35.2%, respectively, P value < .05) than with PROFIT.
On subgroup analysis of patients categorized as high or low risk for SVI, bladder V60, rectum V60, as well as penile bulb mean and V40 remained significantly lower with CHHiP (Tables S3-6).
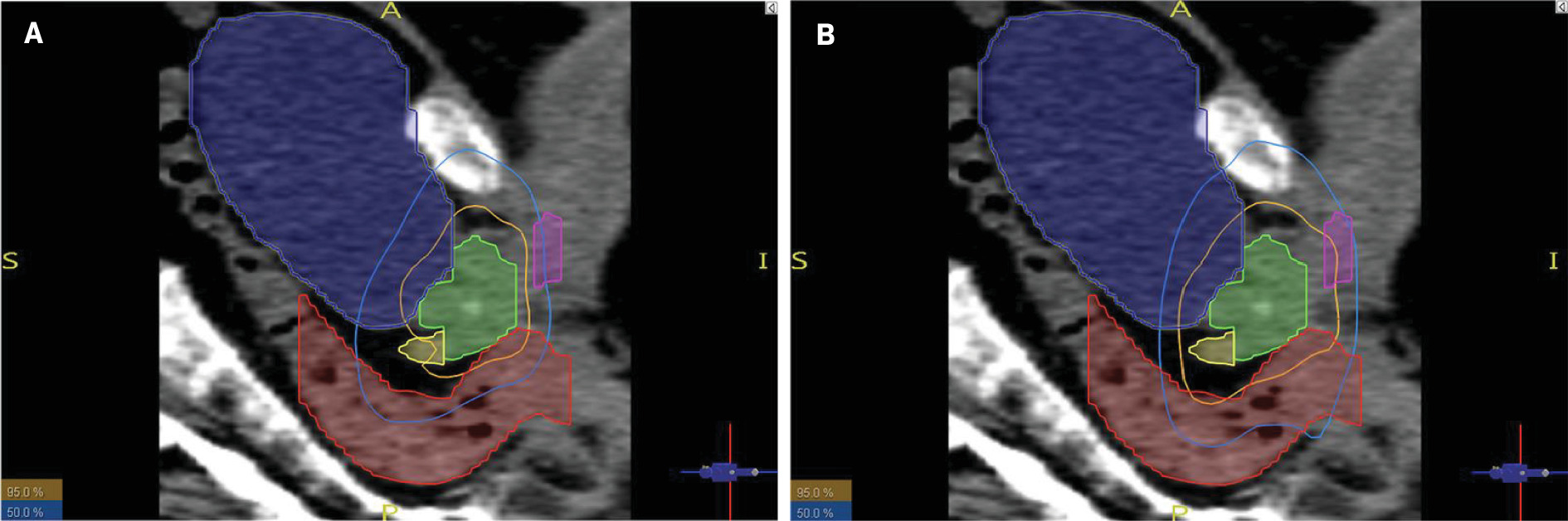
Table 4 summarizes the average and median values of the mean dose, V60, V50, and V40 for each of the OARs in both CHHiP and PROFIT, and a breakdown of all patient doses can be found in the supplementary material (Table S2). Figure 1 illustrates the isodose lines covering the OARs, particularly the penile bulb, for one of the patients (patient #14).
Prostate gland (green), seminal vesicles (yellow), penile bulb (pink), bladder (blue), and rectum (red) in relation to the 95% and 50% isodose lines in CHHiP (A) and PROFIT (B) plans for the same patient (patient #14)
Comparison of the CHHiP and PROFIT Protocols in Terms of Average and Median Values of the Mean Doses to the Bladder, Rectum, and Penile Bulb and in Terms of the Volume Receiving 40 Gy, 50 Gy, and 60 Gy (V40, V50, and V60)
| CHHiP | PROFIT | P VALUE | ||||
|---|---|---|---|---|---|---|
| MEAN (± SD) | MEDIAN | MEAN (± SD) | MEDIAN | |||
| Bladder | Mean (Gy) | 22.7 ± 7.2 | 22.9 | 21.2 ± 10.1 | 19.6 | 0.110 |
| V60 (%) | 1.9 ± 2.0 | 1.1 | 7.7 ± 5.7 | 6.2 | <0.001 * | |
| V50 (%) | 13.2 ± 7.5 | 11.6 | 15.5 ± 10.1 | 12.9 | 0.035 * | |
| V40 (%) | 21.2 ± 10.4 | 18.8 | 21.8 ± 13.4 | 18.1 | 0.753 | |
| Rectum | Mean (Gy) | 27.8 ± 4.5 | 27.2 | 26.0 ± 6.6 | 25.3 | 0.201 |
| V60 (%) | 0.5 ± 0.9 | 0.1 | 4.5 ± 3.2 | 3.8 | <0.001 * | |
| V50 (%) | 13.1 ± 5.6 | 12.3 | 15.7 ± 6.1 | 15.2 | 0.037 * | |
| V40 (%) | 26.4 ± 8.3 | 25.1 | 25.3 ± 10.0 | 22.1 | 0.667 | |
| Penile bulb | Mean (Gy) | 21.9 ± 10.8 | 20.0 | 30.5 ± 12.4 | 27.9 | <0.001 * |
| V60 (%) | <0.1 | <0.1 | 1.3 ± 5.9 | <0.1 | 0.180 | |
| V50 (%) | 5.6 ± 20.5 | <0.1 | 14.4 ± 24.1 | <0.1 | 0.037 * | |
| V40 (%) | 11.4 ± 24.4 | <0.1 | 35.2 ± 31.4 | 30.3 | <0.001 * |
* P values < 0.05 were considered statistically significant as per the related-samples Wilcoxon signed-rank test.
Discussion
In this retrospective dosimetric study comparing doses to OARs, we demonstrated that CHHiP treatment protocol delivers less dose to the bladder, rectum, and penile bulb. The results of this study favor the use of CHHiP protocol over the PROFIT protocol when treating patients with localized, low- to intermediate-risk prostate cancer with moderate hypofractionated external beam radiation therapy.
The radiobiological rationale for using hypofractionation in the treatment of prostate cancer is the low α/β value, which indicates that higher doses per fraction can have a therapeutic advantage while minimizing long-term adverse effects on the surrounding normal tissues.3 Safely decreasing the number of fractions also has logistic and socioeconomic advantages to patients and radiation centers. There have been several trials comparing different dose fractionations to the standard regimens, which use 1.8-2 Gy per fraction. Among these trials are CHHiP and PROFIT.
CHHiP is the largest of these randomized trials that enrolled more than 3000 patients across Europe and New Zealand, and it demonstrated noninferiority of the hypofractionated 60 Gy arm compared with the standard fractionated 74 Gy arm in terms of biochemical and clinical failure-free survival at 5 years (> 90%)6 and at 8 years (>80%).14 The hypofractionated regimen had an acceptable toxicity profile as per the Radiation Therapy Oncology Group (RTOG) scale: the cumulative incidence of grade > 2 bladder and bowel toxicity at 5 years was estimated at 11.7% and 11.9%, respectively.6
PROFIT was also a randomized trial comparing standard and hypofractionated radiation therapy, by enrolling more than 1200 patients from Canada, Australia, and France. It showed a 5-year biochemical/clinical failure disease-free survival of 85% in both arms with no difference in overall RTOG grade > 3 bladder and bowel toxicity. Although gastrointestinal (GI) adverse events grade ≥ 2 were higher in the hypofractionated arm in the acute setting (first 14 weeks), this was reversed in the long term (6 months onward), with late GI toxicity in the hypofractionated arm being lower than in the standard arm (7.4% vs 11%, P value = .006).8
We decided to compare the CHHiP and PROFIT regimens due to identical dose and fractionation in the 2 studies as well as their popularity. PROFIT is more user friendly for both the radiation oncologist and the dosimetrist because there is a single target volume that is treated to a dose of 60 Gy in 20 fractions contrary to the CHHiP protocol where 3 different target volumes exist, requiring a simultaneous integrated boost technique. It may be argued that a comparison between CHHiP and PROFIT cannot be done due to the differential dose distribution in either regimen (ie, CHHiP delivers 60 Gy to PTV1, 57.6 Gy to PTV2, and 48 Gy to PTV3, whereas PROFIT delivers the entire 60 Gy to a single PTV). Both protocols were compared in phase 3 randomized trials to standard fractionation and were shown to be equivalent in terms of oncological outcomes. The question of which shall be preferred in terms of radiation toxicity cannot be answered unless they are compared head-to-head without delineation and dose prescription modifications. To the best of our knowledge, our dosimetric study is the first to compare the two.
Despite moderate hypofractionation becoming standard of care for localized prostate cancer, significant variety exists in target volume definitions in the literature. The American College of Radiology Appropriateness Criteria mentions 2 CTVs in case the risk of seminal vesicle involvement is high (ie, >15%). The first CTV involves both prostate and seminal vesicles, while the second covers the prostate only.15 The French Genito-urinary Group (GETUG) recommendations mention a single CTV, which may include the first centimeter of the seminal vesicles if the tumor is deemed to be high-intermediate risk for SVI.16 Given the different guidelines and recommendations among treatment groups, our comparison is relevant to the current practice of radiation oncology in prostate cancer.
When CHHiP and PROFIT were initiated, image-guided radiation therapy (IGRT) was not yet widespread. When image guidance became more available, the CHHiP trialists started a substudy, where patients either received no IGRT, or IGRT with the original margins, or IGRT with reduced margins: 6 mm/6 mm/3 mm and posteriorly 6 mm/3 mm/0 mm.17 The reduced margins significantly spared the bladder and rectum to a greater degree, as illustrated in lower dose volume and surface percentages ( P value < .0001). Even though radiation oncologists are now inclined to reduce the margins, significant variability still exists in clinical practice. For example, GETUG recommends a margin of 7-10 mm (5-7 mm posteriorly),16 whereas physicians at the Memorial Sloan Kettering Cancer Center suggest margins to be reduced as much as 5 mm (3 mm posteriorly) in a recently published treatment planning guide.18 Similarly, the moderately hypofractionated plans in the PACE-B trial used 5-9 mm margins (3-7 mm posteriorly),19 while those in POP-RT had them at 7 mm (5 mm posteriorly).20 Some protocols require the insertion of fiducial markers, which may not be available in all centers, or the availability of daily cone beam CT imaging, which may be problematic in busy radiation therapy centers with limited resources. As such, in our comparison, we decided to rely on the PTV margins as originally described in the trial protocols.
Our study illustrated that the CHHiP treatment protocol delivered less dose to the bladder, rectum, and penile bulb compared with the PROFIT protocol. While the 2 regimens have not been compared head-to-head in terms of toxicity, we expect this dosimetric benefit to translate into clinical benefit as per dose-toxicity relationships.
In terms of GI toxicity, several reports have correlated the dose received by the rectum in prostate radiation therapy to the incidence of late rectal bleeding. The quantitative analysis of normal tissue effects in the clinic (QUANTEC) review suggested dose constraints in order to decrease the risk of late rectal toxicity of grade ≥ 2 and of grade ≥ 3 to less than 15% and 10%, respectively.21 Accordingly, it was recommended that the rectal volumes receiving 75 Gy, 70 Gy, 65 Gy, 60 Gy, and 50 Gy not exceed 15%, 20%, 25%, 35%, and 50%, respectively. In its recommendations, the QUANTEC review relied on studies where three-dimensional conformal radiation therapy was applied and where radiation was given in standard fractionation. More recently, Wilkins et al presented a dose-volume study that is more applicable to prostate cancer care nowadays.22 Relying on the CHHiP trial, they were the first to derive anorectal dose constraints for hypofractionated IMRT: the volumes receiving 60 Gy, 50 Gy, 40 Gy, 30 Gy, and 20 Gy should be kept below 0.01%, 22%, 38%, 57%, and 85%, respectively. The constraints on the higher doses (40-60 Gy), much tighter than those of QUANTEC, were particularly significant in minimizing rectal bleeding.22 In our comparison, both CHHiP and PROFIT plans met the tighter V50 and V40 constraints suggested by Wilkins et al, but neither satisfied that of V60. That said, V60 in the CHHiP plans was on average 0.5%, which was 9 times less than the average V60 in PROFIT (4.5%). We infer that the CHHiP protocol potentially decreases GI toxicity as opposed to PROFIT, even with the original margins used. We also acknowledge that the plans can be further optimized by using IGRT and reducing the margins, so that all constraints by Wilkins et al may be met and that GI toxicity may be minimized.
Urinary toxicity is also a significant consideration for the quality of life of patients after radiation therapy for prostate cancer, with long-term symptoms including hematuria, dysuria, and increased frequency. However, the QUANTEC review reported that there were no comprehensive data to extract generalizable dose constraints for the bladder.23 With that, it recommended the reliance on the constraints as per the conventional fractionation arm in the noninferiority RTOG 0415 trial, which limited the bladder volume receiving 80 Gy, 75 Gy, 70 Gy, and 65 Gy from exceeding 15%, 25%, 35%, and 50%, respectively.7 Interestingly, for that same trial, a later dose-toxicity analysis found no correlation between the dose received by the bladder and the incidence of genitourinary (GU) toxicity in patients assigned to the hypofractionated arm.24 In our comparison, both CHHiP and PROFIT plans satisfy V48 and V40, but only CHHiP satisfies V60 (1.9% vs 7.7% in PROFIT). While more evidence is surely required to better understand dose and GU toxicity relationships, it is prudent to reduce the dose to the bladder if possible, providing one more reason to favor CHHiP.
The penile bulb is considered a surrogate for neurovascular structures necessary for erectile function, and doses to the penile bulb have been implicated in sexual toxicity. In 2010, the QUANTEC review based on standard fractionated regimens suggested 50 Gy as a threshold mean dose to the penile bulb so as not to increase the risk of impotence post radiation therapy.25 More recently, studies based on hypofractionated regimens have recommended stricter thresholds. In the CHHiP IGRT substudy, researchers found a correlation between the mean and maximum dose to the penile bulb and the incidence of erectile dysfunction.26 With a Royal Marsden Hospital (RMH) grade 2 erectile potency at 2 years as an endpoint, the derived mean dose constraint was 22 Gy, delivered in 3 Gy per fraction, in such a way that the odds of an RMH grade 2 erectile potency were 2.6 times higher in patients whose plans met the constraint than in those whose plans did not. Similar cutoff values have been suggested by a dose-response study from the HYPO-RT-PC trial27 and by another smaller trial from the University of Alabama at Birmingham,28 both of which delivered hypofractionated radiation therapy. In our study, CHHiP plans, which averaged a mean dose of 21.9 Gy, performed significantly better in respecting the stricter threshold (ie, 22 Gy) than the PROFIT plans, which averaged 30.5 Gy. Given the impact of erectile dysfunction on patients’ quality of life, our results once again favor CHHiP.
This study provides the first head-to-head dosimetric comparison between these 2 popular treatment protocols. However, it has several limitations. The IMRT plans generated were step and shoot IMRT and not volumetric-modulated arc therapy. The study was conducted retrospectively in a single tertiary care center on a homogeneous patient population and did not have long-term clinical follow-up to assess for toxicity profiles. It is important to note that these differences in doses delivered to the OARs may not directly correlate to clinical toxicity, especially when the constraints are met. Also, our study is limited to patients with localized low-intermediate-risk disease, and conclusions cannot be extrapolated to other patient populations or to other dose fractionations. Our conclusions may also not apply in case different PTV margins are defined. In other words, our results apply within the scope of the comparison herein, and further studies are needed to corroborate our findings: CHHiP offers superior sparing of the bladder, rectum, and penile bulb compared with PROFIT.
Conclusion
This dosimetric analysis shows that treatment planning with the CHHiP protocol yields lower doses to the bladder, rectum, and penile bulb compared with the PROFIT protocol. These results favor the use of CHHiP as it may decrease the risk of radiation toxicity. Our results need to be validated in a larger cohort of prospectively treated patients.
References
Citation
Paul Ramia, MD, Jana M. Kobeissi, MD, Abbas Mkanna, MS, Bilal Shahine, PhD, Zeinab Makke, MS, Lara Hilal, MD, Dima Mahmoud, MD, Mohammed Mohammed, MD, Farah Olleik, MD, Fady Geara, MD, PhD, Bassem Youssef, MD MBA. CHHiP vs PROFIT for Localized Prostate Cancer: A Retrospective Dosimetric Comparison of Organs at Risk. Appl Radiat Oncol. 2024;(3):24 - 31.
doi:10.37549/ARO-D-24-00017
September 1, 2024