Clinical realization and optimization of MR in radiation therapy
Images

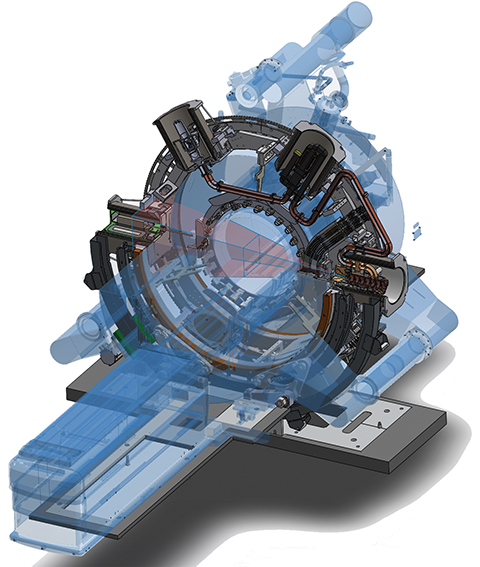
Interest in MR-based radiation therapy (RT) has been mounting over the past few years, and for good reason: MRI offers a host of inherent benefits, namely superior soft-tissue contrast and the ability to derive functional information, such as perfusion and diffusion data, in addition to anatomic imaging details.
“As more precise and hypofractionated techniques are being applied, oncologists are interested in going beyond anatomy with the potential to add biological or functional information, whether that be for contouring the tumor or organs at risk,” says Cecile Mohr, PhD, vice president of marketing and sales for radiation oncology in the Advanced Therapies Business Area at Siemens Healthineers, Malvern, Pennsylvania.
In general, MR provides information on anatomy, tissue function and cellularity, which is particularly important as oncologists and medical physicists seek to adapt RT plans to patient-specific situations and move rapidly toward hypofractionated RT, says Dr. Mohr. Better tumor visualization can potentially reduce volumes while adding functional MR imaging data that paves the way to more personalized treatments and the development of response prediction, she adds. Two functional MR sequences she believes will become invaluable to the oncologist are diffusion-weighted imaging (DWI) and dynamic contrast-enhanced imaging, while other sequences such as spectroscopy (eg, for treatment planning of glioblastomas) are being investigated. Although not yet widely adopted, dedicated MR in RT will enable clinicians to improve patient care and investigate how tumor patterns and functional characteristics based on a multiparametric view of the cancer can be leveraged to improve local control and reduce toxicities, says Dr. Mohr.
While MR imaging has historically been used in RT to complement information obtained by PET/CT and CT simulation by fusing the images together, a change in image-guided RT is on the horizon with two systems/technologies under development: the Elekta MR-linac (Stockholm, Sweden), an investigational high-field MR-adaptive linear accelerator, and the MRIdian linac system (ViewRay, Mountain View, California), pending U.S. FDA 510(k) clearance.
Elekta’s MR-linac
It was once thought nearly impossible to combine a high-field MR system and linear accelerator because the MR magnets would interfere with the linac’s radiation beams and the linac would impact the MRI. However, Elekta and its global collaborators in the MR-linac research consortium have demonstrated the feasibility of this type of system and are in the process of installing the second U.S. and fifth global site at Froedtert & the Medical College of Wisconsin (MCW) Clinical Cancer Center, Milwaukee.
“We essentially have two rooms within the [treatment] room,” explains Kevin Brown, global vice president of scientific research, Elekta. The 1.5T MRI system from Elekta’s technology partner, Royal Philips, lies within the static ring of the linear accelerator. Brown explains that the MR is operationally and magnetically isolated from the linac using active shielding and an RF screen. As the linac rotates in a ring around the MR scanner, the treatment beam passes through the inner MR ring. The radiation beam doesn’t affect the magnetic field of the MRI, and the magnetic field of the MRI doesn’t “scatter” the radiation beam. The MRI can continuously capture images while the beam is on and delivering radiation.
According to Brown, the MRI is comparable to those used in diagnostic radiology and capable of advanced functional sequences such as DWI. The digital linear accelerator is equipped with a multileaf collimator (MLC) and can continuously rotate to deliver advanced therapy techniques. Novel software includes motion management capabilities that use the continuous MR imaging and allow for adaptive planning.
At Froedtert, the department has been preparing for the new MR-linac by optimizing MR sequences and creating the functionality to perform planning based only on MR imaging, says Christopher Schultz, MD, FACR, FASTRO, professor and chairman of Froedtert’s Department of Radiation Oncology.
“Radiation therapy treatment planning currently relies on CT for the tissue density information that planning systems need to display how dose is distributed anatomically in a patient,” Dr. Schultz says. “We are working on methods of assigning densities to the MR images and exploring MR-only workflows to allow for MR-only treatment planning. Such planning methods will be necessary to realize the full potential of MR-guided and adapted radiation therapy.”
Dr. Schultz says MR-linac will be advantageous for treating the liver, pancreas, stomach, and tumors arising in the upper-abdomen in general. “The superior soft-tissue contrast and real-time motion management with MR-linac overcome the limitations of in-room, X-ray-based, image-guidance systems. This functionality will also likely expand use of hypofractionated treatments for prostate, lung and brain tumors or for entirely new sites such as kidney tumors.
“Part of our department’s overarching vision is to use image guidance across the spectrum of malignancy to target tumors with minimal margins [to avoid] adjacent critical structures and decrease toxicity,” he adds. While Dr. Schultz says MR will enable better visualization of soft tissues and organ motion, what’s practice changing is the ability to adapt to these changes daily or weekly as needed. Key to this success is developing software tools and the clinical workflow that allow for timely adaptive changes. He notes that Froedtert’s experience with online adaptive RT using a CT-on-rails RT system, as well as its use of MR imaging in RT, can be directly applied to this project.
“That’s our charge as an early adopter—to define the best practices for safety and quality that need to be hardwired into the workflow,” he adds.
Also of great interest to Dr. Schultz is the use of functional MR sequences, specifically DWI with its ability to create “apparent” diffusion coefficient (ADC) maps for surrogate targeting of the tumor. “When we step back and ask, ‘Why a 1.5T MRI?’ it is to have the whole constellation of diagnostic MR sequences, including functional imaging. So with the Elekta MR-linac, the idea was to develop a combined device without introducing any major compromises to the functionality of a standard 1.5T MR imaging device, and also not make any significant compromises to the linac in terms of functionality and performance. Take the combined device apart and they could still work as fully functional independent devices.”
The MR-linac consortium has identified nine disease sites for which the MR-linac will first be used: the brain, head and neck, esophagus, lung, breast, pancreas, cervix, prostate and rectum. These are areas where the superior soft-tissue contrast of MR and motion management may reduce uncertainty in margins and allow the clinician to more clearly see the borders that will impact treatment volumes and dose.
“This is another enabling technology and a tool in our adaptive treatment paradigm,” adds Dr. Schultz.
Brown is also excited that the MR-linac could reduce uncertainty in planning. He says that cone-beam CT on a linac was a great advance in image-guided therapy, but that an onboard MR is an even bigger breakthrough. “The more we learn about MRI, the more we understand that we can see so much more at the time of treatment,” he says. “ We believe the old way of thinking with uncertainty can be superseded with a new way of thinking: putting the dose just where you want it and nowhere else.”
Next-generation MRIdian Linac
Currently, the only FDA-cleared MR-based RT system is the MRIdian system, which uses cobalt-60 sources to deliver modulated radiation therapy. The works-in-progress MRIdian Linac system builds off this same base system yet replaces the cobalt with a conventional linear accelerator, which also removes a regulatory hurdle, says Michael Saracen, senior director of marketing at ViewRay.
By using technology similar to that in stealth aircraft, ViewRay has engineered a solution that results in no RF impact from the linac on the MRI. This is achieved using patented RF cloaking technology that consists of a copper cylinder lined with carbon fiber. When the linac emits RF noise, the copper reflects the RF and the carbon fiber absorbs it, Saracen explains. Additionally, magnetic shielding technology incorporated around the cylinder creates a “magnetic-free zone” inside the cylinder so it doesn’t impact delivery of the radiation beam during treatment. The linac components are positioned inside these six ferro-magnetic “buckets,” which are mounted to the gantry. Equal distribution of these buckets around the gantry, along with shimming of the magnet, maintains uniformity of the magnetic field. The result is high-quality MR images without distortion due to the radiation beam, he says.
The other important technological development in the MRIdian Linac is the double-focused MLC with two banks of curved leaves that match the divergent angle of the beam. Each photon is either shielded or passes through, significantly reducing the possibility that a photon will leak through. Saracen adds that the radiation beam doesn’t pass through the magnet—there is nothing between the MLC and the patient, which reduces the beam penumbra.
Sasa Mutic, PhD, director of radiation oncology physics at Washington University Siteman Cancer Center, St. Louis, Missouri, visited ViewRay’s corporate offices and worked with the new system using phantoms. As the first site for the MRIdian cobalt-based system, Washington University has used MR-guided adaptive radiation therapy for nearly three years.
“The linac [on MRIdian] offers another advantage of superior dose distributions,” Dr. Mutic says. “There is already a strong indication that the dose distribution with the new system is as good as, if not better than, what we currently have.”
This is particularly important if clinicians want to pursue dose escalation based on the information obtained with MR images for adaptive planning. “Historically, our knowledge of how much dose is delivered to normal structures is poorly understood for many disease sites,” Dr. Mutic says. “Treatment plans are a snapshot of the patient’s anatomy at one point in time, yet it is constantly changing during treatment. What we plan and deliver is often different.”
One capability that Dr. Mutic and his clinic are developing with their MRIdian system is dose recording during treatment delivery. “We can determine how much radiation each organ receives and correlate that to complications,” he says. “To understand how much an organ or tissue can receive, and how much it did receive, personalizes that patient’s treatment and should enable further refinements in radiation therapy.”
MR-guided RT is changing not only how patients are treated but also the type of cases treated, Dr. Mutic says, noting that breast, lung, gastrointestinal and genitourinary cancer are the primary areas treated with the MRIdian at Siteman.
Systems such as MRIdian and MRIdian Linac can also help reduce treatment margins, Dr. Mutic explains. In some patients with favorable anatomic geometry, clinicians can deliver increased doses to the tumor while maintaining dose to critical structures because they can visualize the critical structures at the time of treatment and adapt treatment plans to avoid them. In partial breast irradiation, clinicians at Washington University have reduced the volume of irradiated tissue by > 55%, potentially reducing complications, notes Dr. Mutic.
Thanks to soft-tissue contrast with MRI, it may be possible to treat tumors previously deemed not-treatable due to location in and around organs or critical structures.
“The role of imaging in RT is growing, and there are new innovations that support modern treatment planning,” says Dr. Mohr. “With MRI and other robust technologies, we are able to bring new benefits dedicated to radiation therapy that will support…precision medicine and personalized care.”
Citation
Massat MB. Clinical realization and optimization of MR in radiation therapy. Appl Rad Oncol. 2016;(4):38-41.
December 12, 2016
