Clovis Oncology Opens LuMIERE Clinical Trial for Peptide-targeted Radionuclide Therapy FAP-2286
 The O’Neal Comprehensive Cancer Center at the University of Alabama at Birmingham (UAB) is the first clinical site to open for the Phase 1/2 LuMIERE study of FAP-2286. FAP-2286 is a novel peptide-targeted radionuclide therapy and imaging agent targeting fibroblast activation protein (FAP) from Clovis Oncology, Inc. The O'Neal Comprehensive Cancer Center at UAB is among the nation’s leading cancer research institutions and one of only 51 comprehensive cancer centers designated by the National Cancer Institute.
The O’Neal Comprehensive Cancer Center at the University of Alabama at Birmingham (UAB) is the first clinical site to open for the Phase 1/2 LuMIERE study of FAP-2286. FAP-2286 is a novel peptide-targeted radionuclide therapy and imaging agent targeting fibroblast activation protein (FAP) from Clovis Oncology, Inc. The O'Neal Comprehensive Cancer Center at UAB is among the nation’s leading cancer research institutions and one of only 51 comprehensive cancer centers designated by the National Cancer Institute.
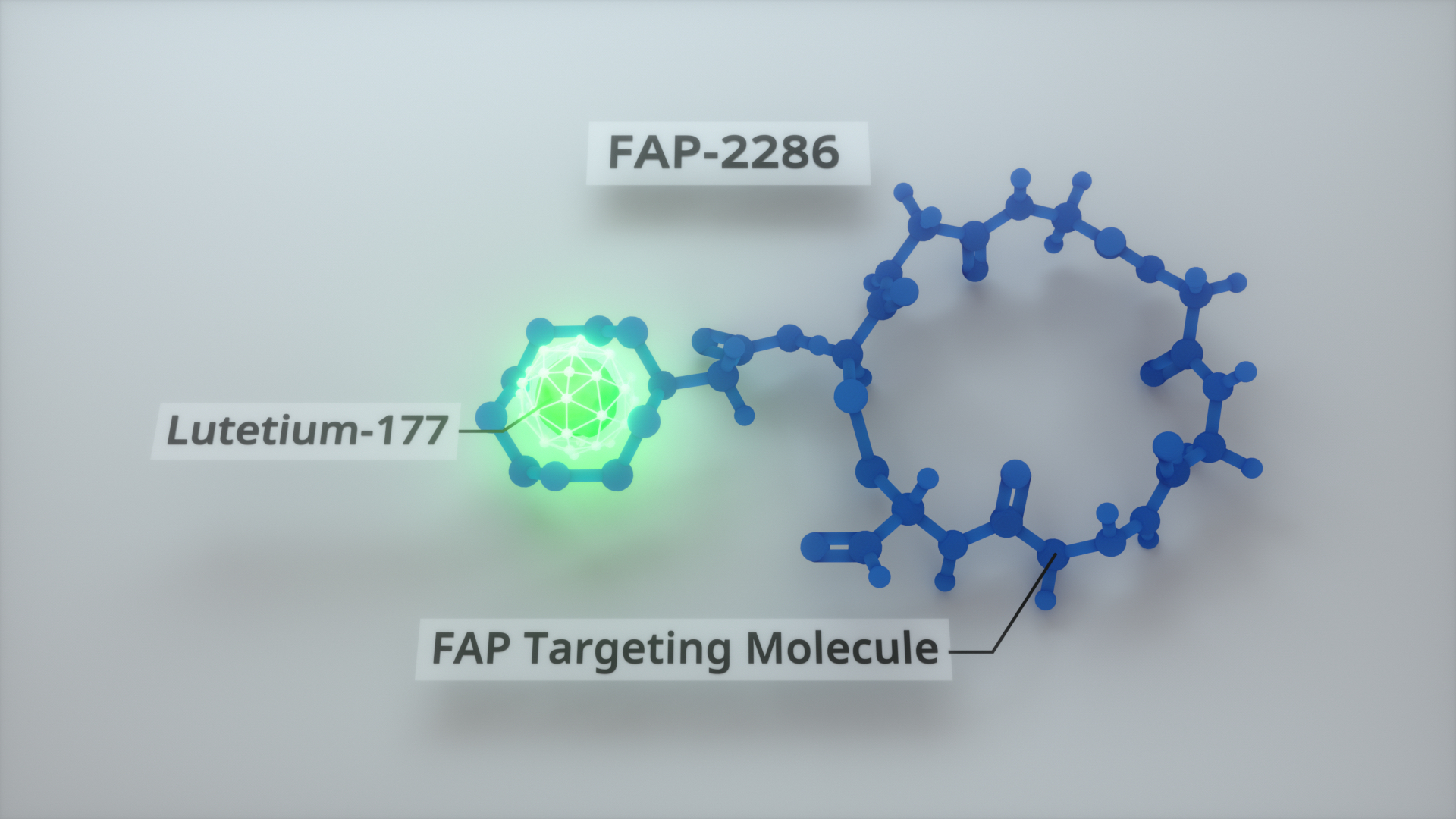
The Phase 1 portion of the LuMIERE study will evaluate the safety of the FAP-targeting investigational therapeutic agent and identify the recommended Phase 2 dose and schedule of lutetium-177 labeled FAP-2286 (177Lu-FAP-2286). FAP-2286 labeled with gallium-68 (68Ga-FAP-2286) will be utilized as an investigational imaging agent to identify patients with FAP-positive tumors appropriate for treatment with the therapeutic agent. Once the Phase 2 dose is determined, Phase 2 expansion cohorts are planned in multiple tumor types.
“I envision that targeted radionuclide therapy has the potential to transform how we diagnose and treat cancer and I look forward to exploring this in the LuMIERE clinical trial,” said Thomas Hope, M.D., Director of Molecular Therapy in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco and lead investigator of the LuMIERE trial.
FAP is a cell-surface protein that is expressed in limited amounts by normal tissues, but highly expressed in cancer-associated fibroblasts (CAFs) present in the tumor microenvironment of many solid tumors including breast, lung, colorectal and pancreatic carcinomas.1-4 Preclinical data demonstrate that 177Lu-FAP-2286 potently and selectively binds FAP on the surface of CAFs and tumor cells to deliver the beta particle-emitting radioisotope 177Lu, resulting in DNA damage and cell death.4 Compelling anti-tumor efficacy of 177Lu-FAP-2286 has been demonstrated in FAP-expressing preclinical tumor models.4
FAP-2286 consists of two functional elements; a targeting peptide that binds to FAP and a site that can be used to attach radioactive isotopes for imaging and therapeutic use. FAP is highly expressed in many epithelial cancers, including more than 90 percent of breast, lung, colorectal and pancreatic carcinomas. FAP-2286 is an unlicensed medical product.
“We are pleased to initiate sponsored clinical development of FAP-2286 with the LuMIERE study based on the clinical community’s enthusiasm to further explore the potential of targeted radionuclide therapy and FAP as a therapeutic target,” said Patrick J. Mahaffy, President and CEO of Clovis Oncology. “Given FAP is highly expressed in many of the hardest-to-treat solid tumors, we look forward to exploring the potential of FAP-2286 to treat patients with cancer as our first entry into this emerging field of targeted radiotherapy. The O’Neal Comprehensive Cancer Center and each of the clinical trial sites expected to open for enrollment in the near future bring tremendous nuclear medicine and medical oncology expertise as well as passion for the program.”