Disease Characteristics, Treatment Tolerance, and Toxicity in Older Patients (≥ 65 y) With Oropharyngeal Cancer and Implications
Affiliations
- 1 Department of Radiation Oncology, Henry Ford Health-Cancer, Detroit MI
- 2 Clinical Oncology Department, Faculty of Medicine, University of Alexandria, Alexandria, Egypt
- 3 Department of Public Health Sciences, Henry Ford Health, Detroit MI
- 4 College of Human Medicine, Michigan State University, Lansing MI
- 5 Department of Otolaryngology, Henry Ford Health, Detroit MI
Objective:
To assess and compare tolerability for standard-of-care treatments and evaluate outcomes in older and younger patients with oropharyngeal cancer (OPC).
Methods and Materials:
We queried our institutional database for nonmetastatic OPC treated curatively between January 2009 and June 2020, with radiation therapy ± systemic therapy, or surgery ± adjuvant radiation therapy ± systemic therapy. We compared clinicopathological, treatment-related, and therapeutic toxicity features, and survival outcomes between older (≥ 65 y at diagnosis) and younger (< 65 y at diagnosis) patients across human papilloma virus (HPV) subtypes. Multivariate analyses for predictors of survival in all patients were performed.
Results:
In this retrospective study, we evaluated 340 patients with OPC: 123 (36%) older and 217 (64%) younger. There were 252 patients (74%) with HPV+ve OPC. The HPV+ve older patients showed an increasing trend over the years studied. Definitive radiation therapy ± systemic therapy was utilized in 73.2%, while the remainder had surgery ± adjuvant radiation therapy ± systemic therapy. After a median follow-up of 5.24 (interquartile range: 3.53) years, no significant differences in treatment received, overall, disease-free, locoregional recurrence-free, or distant metastasis-free survival were seen between age groups, regardless of HPV status. Significantly larger proportions of older patients received cetuximab (25.8% vs 11.9%; P < .001), required hospitalization (46.6% vs 26.9%; P < .001), required feeding tubes (63.6% vs 49.5%; P = .02), and were switched to a less-toxic systemic therapy protocol (18.3% vs 7.7%; P = .019). For all patients, factors such as radiation therapy course completion, radiation therapy delays, unplanned hospitalizations, and feeding tubes never removed were independently associated with various survival endpoints.
Conclusion:
While survival outcomes were equivalent between older and younger patients with OPC, older patients exhibited lower tolerance and higher toxicity from systemic therapy, suggesting a need for enhanced multidisciplinary supportive care including geriatric assessment, for older patients receiving concomittant radiation and systemic therapy.
Introduction
Oropharyngeal squamous cell carcinoma (OPC) is one of the most common head and neck cancers (HNCs), with a median age at diagnosis of 60 to 63 years and over 21,000 new cases expected in 2024, with more than a one-third in adults 65 years or older.1, 2 Unlike other smoking-related tumors, which are on the decline, OPC incidence has been significantly rising, driven mainly by human papillomavirus-related (HPV+ve) tumors.3 This rising trend is highest in adults > 65 years old, especially white males,4 and within the context of an aging population,5 over 18,000 cases of OPC in older adults are expected by 2030, forming 60% of total cases, almost double the 9270 cases diagnosed in 2019.2, 6
Notably, the profile for an HPV+ve OPC patient as a young, white, fit male7, 8 has been shifting with the median age at diagnosis recent increases, which means we are currently facing an evolving epidemic of OPC in older adults driven mainly by HPV+ve tumors.9, 10 It has been demonstrated that older patients with HPV+ve have better prognoses than HPV-unrelated (HPV-ve) OPC.11 - 13 With a growing population of survivors, understanding how current OPC therapeutic approaches affect this group in regard to quality of life (QOL) becomes more critical.
Older adults are vastly underrepresented in cancer clinical trials, and there is concern that standard-of-care (SoC) regimens validated in predominantly younger populations may not be directly applicable to older patients with OPC.14, 15 Thus, considering the rising age of patients with OPC, a critical question remains as to whether older patients may tolerate current SoC therapies differently than younger patients. This potential disparity underscores the need for tailored approaches that consider age-related physiological changes and comorbidities. Key practice-changing studies have highlighted a nonsignificant survival benefit in older patients from concomitant chemotherapy, in the definitive/adjuvant radiation therapy (RT) settings, and also for concomitant targeted therapy.16 - 18 Importantly, the cohorts of these studies contained a low proportion of older adults (< 5%) and were not prospectively powered for age. Other reports have also shown worse outcomes in older patients with OPC, even after accounting for stage and type of treatment.3, 19, 20 While some studies have shown more RT-related hospitalizations, treatment interruptions, and feeding tube dependence for older patients,21, 22 others have shown equivalent treatment tolerance between age groups.23, 24 However, many studies have not accounted for HPV status , included non-OPC cases, or used older/nonstandard RT techniques and systemic therapy (ST) protocols.
Intriguingly, more recent reports have shown a survival gain from multimodality treatment in older patients with accepted treatment tolerance.13, 25 - 28 Nevertheless, these studies lacked a comparative group with younger patients, highlighting a knowledge gap in our understanding of how OPC therapeutic strategies may affect older adults for outcomes, toxicity, and tolerability.
Considering the conflicting results and variable designs of previous studies, we sought to define the clinicopathological characteristics and treatment tolerability/toxicity profiles of older (≥ 65 y) vs younger (< 65 y) patients with OPC. We aimed to conduct a comprehensive analysis to determine the correlation between age, HPV status, and treatment approaches, with outcomes in patients with OPC who had been treated curatively.
Methods and Materials
Study Population
Following institutional review board approval, we queried our institutional HNCs database for patients who had nonmetastatic primary OPC diagnosed between January 2009 and June 2020. All patients had HPV status determined via immunohistochemistry p16 protein testing of tumor biopsies.29, 30 Patients were excluded if they had: HPV+ve tumors in neck lymph nodes (LNs) with no primary OPC; HPV+ve tumors in which the majority of the tumor was in the oral cavity or larynx; or previous RT to the head-and-neck region. Patients who did not receive curative treatment, those referred to another institution, and those with inadequate follow-up were also excluded.
Treatment and Follow-Up
All patients underwent baseline performance and cancer staging assessments, and all were presented and discussed at multidisciplinary HNCs tumor board meetings. Treatment approaches were based on tumor stage, subsite, and HPV status. Curative treatments included definitive RT (± concomitant ST) or surgery, mainly transoral resection (TORS) ± LN dissection followed by adjuvant RT (± ST), depending on pathological risk factors.
Patients were assessed weekly during RT and toxicities were graded per CTCAE v4.0. Nutritional assessment was performed by a registered dietitian. Following treatment, patients had follow-up visits every 3-to-4 months for 2 years; every 4-to-6 months for 3 years; and annually thereafter, with imaging studies ordered as appropriate.
Study Variables and Treatment Groups
Sociodemographic and clinicopathological characteristics, treatment received, recurrence events (local, regional/nodal, distant), and survival status at the last follow-up were recorded. Comorbidity at diagnosis was calculated using Charlson comorbidity index (CCI).31 Patients diagnosed before 2018 were re-staged per the criteria of the American Joint Committee on Cancer (AJCC) 8th edition.32
Patients were divided into 2 groups: an older group (≥ 65 y) and a younger group (< 65 y) based on age at OPC diagnosis. The 65-year cutoff, that allowed robust statitical comparison, was chosen based on the US Census Bureau guidelines5 and other important studies.6, 10, 15, 27, 33, 34 Study variables and survival outcomes were compared between older and younger groups stratified by HPV status. Trends for new cases in the study years were also assessed by age.
Survival Endpoints
Primary survival endpoints included overall survival (OS), locoregional recurrence-free survival (LRFS), distant metastases-free survival (DMFS), and disease-free survival (DFS), which was recorded as the date of first recurrence or death. Local and regional/nodal recurrence-free survival was also assessed. The date of biopsy was the starting timepoint for all endpoints.
Treatment Tolerance and Toxicity
RT Duration and Delays
RT interruptions were assessed, including prescribed RT course not completed and unplanned RT delays beyond 2 days. Treatment package time (TPT), days between surgery dates and last RT fraction for patients receiving adjuvant RT, and total RT duration, days between first and last RT fractions, were calculated.
Acute Toxicity from RT and Nutritional Support
RT toxicity was measured according to factors such as worst grade of acute mucositis, worst pain score, unplanned hospitalization (excluding admission for intravenous hydration), maximum weight loss, and need for feeding tube (percutaneous endoscopic gastrostomy [PEG] and/or nasogastric tube). At our institution, we do not place feeding tubes prophylactically. Patients experiencing severe dysphagia/odynophagia that prevented adequate oral intake, resulting in significant weight loss and/or dehydration, received a feeding tube following a multidisciplinary evaluation by a speech-language pathologist, dietitian, and radiation oncologist. We calculated total days of feeding tube dependency and reported patients who never had their tubes removed (long-term feeding tube until death/last follow-up).
Systemic Therapy Tolerance and Toxicity
ST protocol received, including cumulative cisplatin dose (mg/m2 ), was reported. Dose reduction for a received cycle, unplanned delays, febrile neutropenia events, canceled doses, and rates of switching to a less intense ST protocol due to toxicity was compared between age groups.
Statistical Analysis
Descriptive statistics were reported as frequencies (%) for categorical variables and median (range) for continuous variables. Age groups stratified by HPV status were compared with chi-square test or Fisher exact tests for categorical variables and Wilcoxon rank sum test for continuous variables. Kaplan-Meier curves were plotted, and log-rank tests were conducted to compare survival endpoints. Univariate analysis (UVA) and multivariate (MVA) Cox regression models were used to identify independent predictors for survival. For MVA, if abundant predictors relative to small event numbers were present, backward stepwise selection was applied to minimize potential collinearity and overfitting. All tests were 2-sided, and statistical significance was set to P ≤ .05. All analyses were performed in R 4.2.1.
Results
Demographic, Clinicopathological, and Treatment Characteristics
A total of 340 patients with OPC were included: overall median age was 61 (range, 38-91) years; 284 (83.5%) men. There were 252 (74%) patients with HPV+ve and 88 (26%) with HPV-ve OPC. Most tumors were located in the tonsil (44%) or base of tongue (36%), and most patients had stage 1 (43%) or 2 (19%) cancer. The majority of patients ( n = 249; 73%) received definitive RT ± ST, and 91 patients (27%) had TORS with or without adjuvant RT±ST.
Age Groups and Trends Over Time
A total of 123 patients (36%) were in the older group: 90 with HPV+ve and 33 with HPV-ve OPC. There were 217 (64%) patients in the younger group: 162 with HPV+ve and 55 with HPV-ve OPC. HPV+ve proportion was nonsignificant across age groups. The proportion of older patients increased over time, in which 33% treated from 2009 to 2012 were older compared with 38% for 2017-2020, with a parallel trend for HPV+ve cases ( Figure 1 ). There was a significant increase over time in the older group with HPV+ve OPC (60% [2009-2012] vs 84.1% [2017-2020]; P = .019).
Trends for newly diagnosed patients over the periods of diagnosis for all ( N = 340), HPV+ve ( N = 252), and older patients with oropharyngeal cancer ( N = 123).
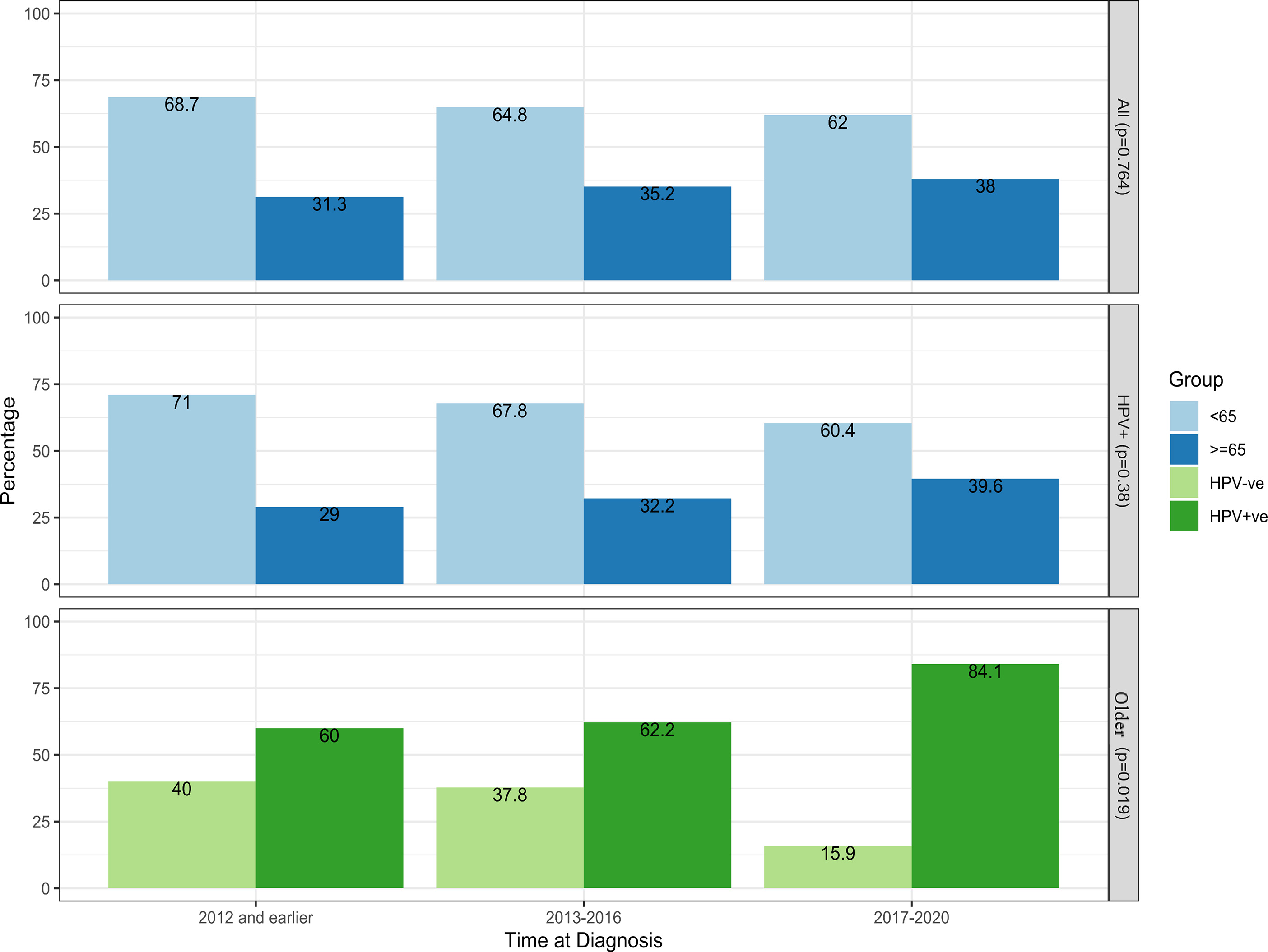
Age groups were generally similar; however, median CCI was significantly higher in older patients ( P = .033). The older group had a higher smoking history among HPV-ve and a trend for more base of tongue tumors in HPV+ve patients. All detailed descriptive characteristics by age and HPV status are presented in Table 1 , and Supplementary Table 1 ( www.appliedradiationoncology.com ).
Baseline Demographic, Clinicopathological, and Treatment Characteristics for Older ( ≥ 65 y) vs Younger Patients With Oropharyngeal Cancer Stratified by Human Papillomavirus Status
| All patients ( N = 340) | HPV+ve OP ( N = 252) | P value | HPV-ve OPC ( N = 88) | P value | |||
|---|---|---|---|---|---|---|---|
| Characteristics | Older (≥ 65 y) ( N = 90) (35.7%) | Younger (<65 y) ( N = 162) (64.3%) | Older (≥ 65 y) ( N = 33) (37.5%) | Younger (<65 y) ( N = 55) (62.5%) | |||
| Age, y, median (range) | 61 (38-91) | 70 (65-91) | 56 (38-64) | <.001 | 68 (65-85) | 57 (38-64) | <.001 |
| CCI, median (range) | 1 (0-8) | 1 (0-8) | 0 (0-8) | .005 | 1 (0-6) | 1 (0-5) | .033 |
| Sex, N (%) | .377 | .765 | |||||
| Male | 284 (83.5) | 77 (85.6) | 146 (90.1) | 24 (72.7) | 37 (67.3) | ||
| Female | 56 (16.5) | 13 (14.6) | 16 (9.9) | 9 (27.3) | 18 (32.7) | ||
| Race, N (%) | .89 | .286 | |||||
| Black/African American | 65 (19.1) | 11 (12.2) | 23 (14.2) | 12 (36.4) | 19 (34.5) | ||
| White | 261 (76.8) | 75 (83.3) | 133 (82.1) | 18 (54.5) | 35 (63.6) | ||
| Other | 14 (4.1) | 4 (4.4) | 6 (3.7) | 3 (9.1) | 1 (1.8) | ||
| Marital status, N (%) | .028 | .001 | |||||
| Married | 207 (60.9) | 52 (57.8) | 112 (60.9) | 13 (39.4) | 30 (54.5) | ||
| Single | 82 (24.1) | 19 (21.1) | 35 (21.6) | 7 (21.2) | 21 (38.2) | ||
| Divorced/widowed | 51 (34) | 19 (21.1) | 15 (9.3) | 13 (39.4) | 4 (7.3) | ||
| Alcohol use, N (%) | .76 | .576 | |||||
| Social/occasional | 151 (44.4) | 43 (47.8) | 81 (50) | 10 (30.3) | 17 (30.9) | ||
| Frequent/alcoholism | 93 (27.4) | 17 (18.9) | 34 (21) | 14 (42.4) | 28 (50.9) | ||
| Smoking status, N (%) | .652 | .007 | |||||
| Current smoker | 82 (24.1) | 13 (14.4) | 28 (17.3) | 12 (36.4) | 29 (52.7) | ||
| Former smoker | 147 (43.2) | 43 (47.8) | 68 (42) | 20 (60.6) | 16 (29.1) | ||
| Never smoker | 111 (32.6) | 34 (37.8) | 66 (40.7) | 1 (3) | 10 (18.2) | ||
| Smoking pack-years, median (range) | 30 (1-160) | 24 (1-160) | 20 (1-150) | .246 | 40 (10-120) | 35 (8-140) | .014 |
| Marijuana use, N (%) | 55 (16.2) | 10 (11.2) | 32 (19.8) | .112 | 2 (6.1) | 11 (20) | .119 |
| Oropharyngeal subsite, N (%) | .061 | .939 | |||||
| Tonsil | 148 (43.5) | 36 (40) | 91 (56.2) | 9 (27.3) | 12 (21.8) | ||
| Base of tongue | 122 (35.9) | 40 (44.4) | 54 (33.3) | 10 (30.3) | 18 (32.7) | ||
| Soft palate/pharyngeal wall | 22 (6.5) | 2 (2.2) | 5 (3.1) | 5 (15.1) | 10 (18.2) | ||
| Multiple sites | 48 (14.1) | 12 (13.3) | 12 (7.4) | 9 (27.3) | 15 (27.3) | ||
| AJCC stage 8th, N (%) | .171 | .284 | |||||
| 1 | 146 (42.9) | 45 (50) | 94 (58) | 3 (9.1) | 4 (7.3) | ||
| 2 | 63 (18.5) | 21 (23.3) | 41 (25.3) | 0 (0) | 1 (1.8) | ||
| 3 | 60 (17.6) | 24 (26.7) | 27 (16.7) | 1 (3) | 8 (14.5) | ||
| 4A | 54 (15.9) | N/A | N/A | 24 (72.7) | 30 (54.5) | ||
| 5B | 17 (5) | N/A | N/A | 5 (15.2) | 12 (21.8) | ||
| Overall treatment, N (%) | .307 | .655 | |||||
| Surgery alone | 14 (4.1) | 3 (3.3) | 5 (3.1) | 2 (6.1) | 4 (7.3) | ||
| Surgery + adjuvant RT | 32 (9.4) | 10 (11.1) | 17 (10.5) | 3 (9) | 2 (3.6) | ||
| Surgery + adjuvant CRT | 45 (13.2) | 7 (7.8) | 28 (17.3) | 2 (6.1) | 8 (14.5) | ||
| Definitive RT | 33 (9.7) | 6 (6.7) | 12 (7.4) | 6 (18.2) | 9 (16.4) | ||
| Definitive CRT | 216 (63.5) | 64 (71.1) | 100 (61.7) | 20 (60.6) | 32 (58.2) | ||
| Positive final surgical margin, N (%) | 23 (25.3) | 7 (35) | 9 (18) | .224 | 2 (28.6) | 5 (35.7) | .52 |
| Lymphovascular invasion, N (%) | 46 (50.5) | 9 (45) | 24 (48) | >.99 | 6 (85.7) | 7 (50) | .174 |
| Perineural invasion, N (%) | 13 (14.3) | 1 (5) | 7 (14) | .424 | 0 (0) | 5 (35.7) | .124 |
| Extracapsular nodal extension, N (%) | 31 (33) | 5 (26.3) | 16 (30.2) | .98 | 3 (42.9) | 7 (46.7) | > .99 |
| Period of diagnosis, N (%) | .38 | .654 | |||||
| 2009-2012 | 46 (13.5) | 9 (10) | 22 (13.6) | 6 (18.2) | 9 (16.4) | ||
| 2013-2016 | 128 (37.6) | 28 (31.1) | 59 (36.4) | 17 (51.5) | 24 (43.6) | ||
| 2017-2020 | 166 (48.8) | 53 (58.9) | 81 (50) | 10 (30.3) | 22 (30.3) |
Bold values are statistically significant values.
Abbreviations : AJCC, American Joint Committee on Cancer; CCI, Charlson Comorbidity Index; CRT, concomitant chemoradiotherapy; HPV+ve, human papilloma virus-related; HPV-ve, human papilloma virus-unrelated; OPC, oropharyngeal squamous cell carcinoma; RT, radiation therapy.
Survival by Age and HPV Status
After a median follow-up time of 4.9 (interquartile range [IQR]: 3.2) years for older and 5.3 (IQR: 3.8) years for younger patients, P = 0.35, 124 died (56.5% with recurrence and 43.5% due to other causes) and 92 recurred: 54 local (16%), 34 nodal/regional (10%), and 46 distant (13.5%) most frequently in the lung (52.2%). No treatment-related deaths during RT occurred.
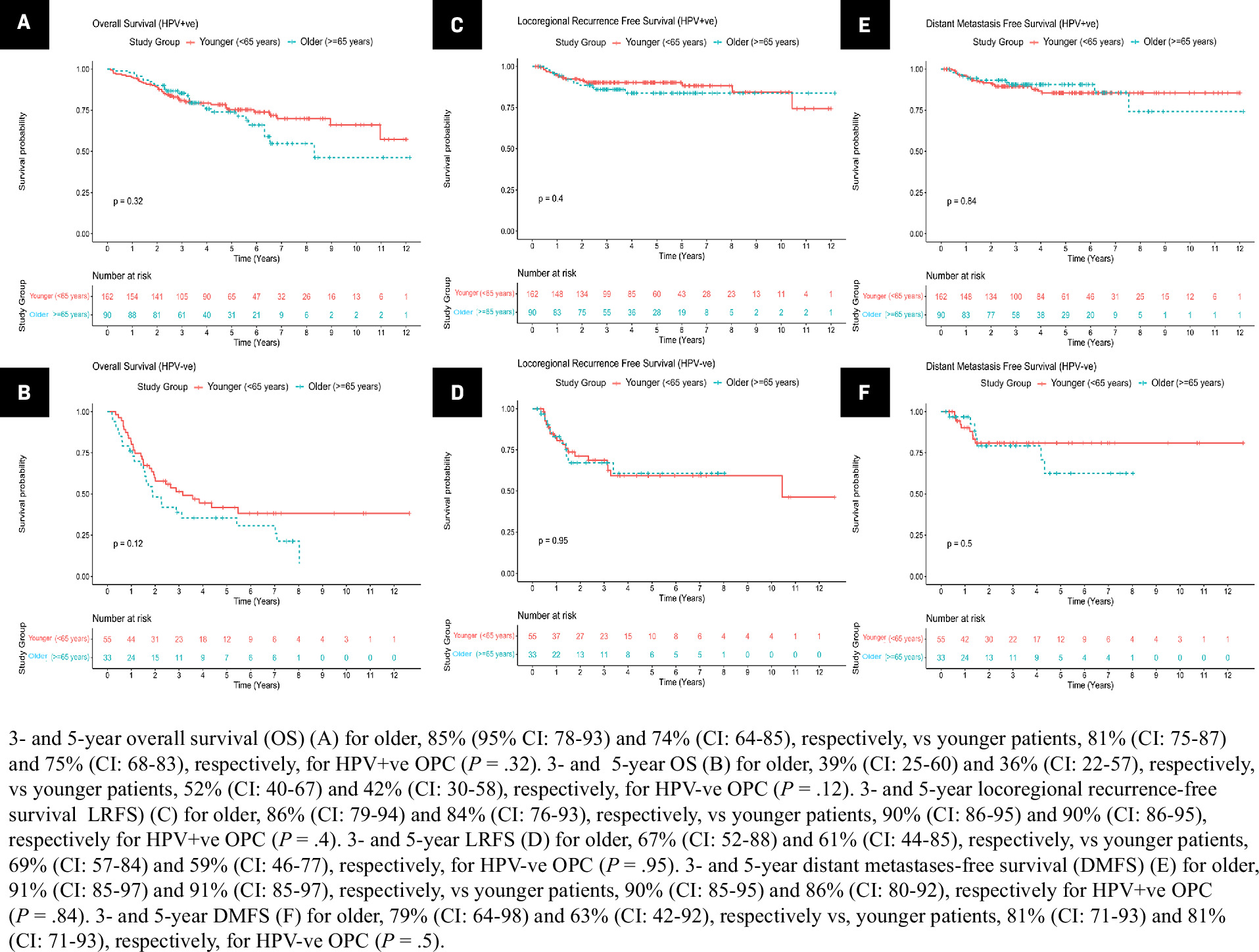
We observed no significant differences in 3- and 5-year OS, LRFS, or DMFS between older and younger patients for both HPV subtypes ( Figure 2A-F ). Neither DFS nor local and nodal/regional recurrence-free survival was significantly different between age groups regardless of HPV status ( Supplementary Figure 1A-B,2A-B,3A-B , respectively; ( www.appliedradiationoncology.com ). Sites of distant metastases were also not significantly different with age ( Supplementary Figure 1, www.appliedradiationoncology.com ).
Kaplan-Meier estimates of survival endpoints for the study cohort ( N = 340) in older (≥ 65 y) vs younger (< 65 y) patients with log-rank test within HPV+-ve and HPV-ve oropharyngeal cancer (OPC).
Multivariate Analysis: Survival by Age and HPV Status
Based on findings from UVA, Cox regression MVA identified factors associated with survival outcomes for older patients ( N = 123) ( Table 2 ). Notably, HPV status was associated with all endpoints (HR 1.67-2.97); and CCI and tumor stage were significant predictors for OS. Marital status, OPC subsite, and overall treatment received were prognostic for DFS, while advanced AJCC stage and diagnosis in earlier years were associated with worse DMFS.
Multivariate Analysis of Variables Associated With Survival in Older Patients (≥65 y) With Oropharyngeal Cancer
| Variable | Older Patients ≥ 65 y With Oropharyngeal Cancer ( N = 123) | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall survival | Disease-Free Survival | Locoregional Recurrence-Free Survival | Distant Metastasis-Free Survival | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| HPV status (-ve vs +ve) | 1.84 (1.27- 2.67) a |
.001 | 1.67 (1.23- 2.26) b 1 |
.001 | 2.97 (1.27- 6.92) c |
.012 | ** | N/A |
| Age (continuous) | 1.04 (0.98- 1.11) |
.174 | ** | N/A | 0.91 (0.81- 1.01) |
.071 | ** | N/A |
| CCI (continuous) | 1.25 (1.02- 1.53) |
.028 | ** | N/A | ** | N/A | ** | N/A |
| Marital status (divorced/widowed vs single) | ** | N/A | 2.7 (1.1- 6.57) b 1 |
.028 | ** | N/A | ** | N/A |
| Oropharyngeal subsite (soft palate/pharyngeal wall vs tonsil) | 2.36 (0.84- 6.67) |
.105 | 2.97 (1.05- 8.43) b 2 |
.04 | ** | N/A | ** | N/A |
| Tumor stage | ||||||||
| T2 vs T1 | 1.92 (0.71- 5.23) |
.2 | 0.93 (0.4- 2.16) b 1 |
.862 | ** | N/A | ** | N/A |
| T3 vs T1 | 3.67 (1.07- 12.6) |
.039 | 1.79 (0.63- 5.07) |
.274 | ** | N/A | ** | N/A |
| T4a/NOS vs T1 | 3 (1.12- 8) |
.028 | 1.33 (0.55- 3.25) |
.527 | ** | N/A | ** | N/A |
| T4b vs T1 | 12.9 (1.95- 85) |
.008 | 4.62 (0.98- 21.8) |
.053 | ** | N/A | ** | N/A |
| AJCC stage 8th (4B vs 1) | ** | N/A | ** | N/A | 1 (0.13- 7.61) |
.99 | 16.9 (2.91- 97.6) d 1 |
.002 |
| Total LN dissected (continuous) | 0.98 (0.97- 1) |
.097 | ** | N/A | ** | N/A | ** | N/A |
| ST received | ||||||||
| Cetuximab vs platinum | 1.64 (0.74- 3.67) |
.224 | 3.96 (1.89- 8.29) b 2 |
<.001 | ** | N/A | 1.77 (0.48- 6.53) d 2 |
.392 |
| No ST vs platinum | 0.91 (0.34- 2.43) |
.848 | 1.27 (0.59- 2.71) |
.543 | ** | N/A | 0.63 (0.13- 3.1) |
.568 |
| Overall treatment | ||||||||
| Surgery alone vs definitive CRT | ** | N/A | 4.23 (1.15- 15.6) b 1 |
.03 | ** | N/A | ** | N/A |
| Surgery + adj RT vs definitive CRT | ** | N/A | 0.30 (0.1- 0.95) |
.04 | ** | N/A | ** | N/A |
| Surgery + adj CRT vs definitive CRT | ** | N/A | 0.63 (0.19- 2.01) |
.432 | ** | N/A | ** | N/A |
| Definitive RT vs definitive CRT | ** | N/A | 1.25 (0.47- 3.3) |
.651 | ** | N/A | ** | N/A |
| Period of diagnosis (2017-2020 vs 2009-2012) |
** | N/A | ** | N/A | ** | N/A | 0.15 (0.03- 0.66) d 1 |
.013 |
Bold values are statistically significant values; ** not included in the mutivariate analysis model
Abbreviations: Adj, adjuvant; AJCC, American Joint Committee on Cancer; CCI, Charlson Comorbidity Index; CRT, concomitant chemoradiotherapy; HR, hazard ratio; HPV+ve, human papilloma virus-related; HPV-ve, human papilloma virus-unrelated; LN, lymph node; N/A, not applicable; RT, radiation therapy; ST, systemic therapy.
aOverall survival for older patients adjusted for alcohol use, smoking status, surgery, RT, and era of diagnosis.
bDisease-free survival models of older patients:1 adjusted for surgical margin and oropharyngeal subsite,2 adjusted for HPV status, marital status, overall T stage (excluding overall treatment).
cLocoregional recurrence-free survival for older patients adjusted for smoking status, total LN dissected, and RT.
dDistant metastases-free survival models for older patients:1 adjusted for total positive LN, and HPV status,2 adjusted for period of diagnosis and AJCC stage (8th).
We also characterized predictors of survival based on HPV status for the entire cohort ( N = 340), adjusting for age ( Supplementary Table 2, www.appliedradiationoncology.com ). For patients with HPV+ve OPC, the major predictors for OS included CCI (HR 1.42 [CI: 1.25-1.61]), marijuana use (HR 2.66 [CI: 1.39-5.07]), and AJCC stage 3 vs 1 (HR 2.76 [CI: 1.57-4.84]). AJCC stage, adjuvant vs definitive RT, and period of diagnosis were independently associated with LRFS; smoking pack-years and extracapsular nodal extension (ENE) were deterministic for DMFS, whereas for HPV-ve patients, smoking status, tumor stage, and ST protocol were associated with OS and DFS. Cetuximab was inferior to platinum protocols for all endpoints for both HPV subgroups.
Treatment Tolerability and Acute Toxicity During RT
The central objective of our study was to investigate potential differences in therapeutic details and toxicity outcomes in older vs younger patients who had received RT for OPC ( N = 326). The age groups were similar for most of these parameters ( Table 3 ); however, a significantly higher proportion of older patients were hospitalized during RT (46.6% vs 26.9%; P < .001), received a feeding tube during RT (63.6% vs 49.5%; P = .02), and had their feeding tube never removed (19.5% vs 8.7%; P = .008). RT interruptions, total RT duration, as well as TPT were nondifferent with age.
Radiation Therapy and Systemic Therapy Parameters, and Acute Toxicity Outcomes for Older (≥65 y) vs Younger Patients With Oropharyngeal Cancer Who Received Radiation Therapy ( N = 326)
| Characteristic | Older OPC (≥65 y) ( N = 118) (36.2%) |
Younger OPC (<65 y) ( N = 208) (63.8%) |
P value |
|---|---|---|---|
| RT course not completed | 5 (4.2) | 9 (4.3) | >.99 |
| Unplanned RT delays a | 29 (25.7) | 42 (21.1) | .434 |
| Total RT duration, days, median (range) b | 49 (41-71) | 49 (36-76) | .311 |
| TPT, days, median (range) c | 86 (72-128) | 81 (65-137) | .265 |
| TPT > 90 d c | 8 (38.1) | 15 (28.3) | .588 |
| TPT > 100 d c | 4 (19.0) | 9 (17.0) | >.99 |
| Acute grade 3 mucositis | 52 (44.1) | 87 (41.8) | .742 |
| Worst grade of pain during RT, median (range) | 7 (0-10) | 7 (2-10) | .792 |
| Worst pain grade ≥ 8 | 38 (32.2) | 69 (33.2) | .955 |
| Unplanned hospitalization during RT d | 55 (46.6) | 56 (26.9) | <.001 |
| Unplanned IV hydration during RT e | 40 (33.9) | 70 (33.7) | >.99 |
| Weight loss during RT | 107 (90.7) | 192 (92.3) | .761 |
| Weight loss during RT > 10% | 51 (43.2) | 94 (45.2) | .819 |
| Feeding tube during RT | 75 (63.6) | 103 (49.5) | .02 |
| Feeding tube inserted before RT start f | 23 (19.5) | 23 (11.1) | .28 |
| Feeding tube inserted after RT start (reactive) | 52 (44.1) | 80 (38.5) | .28 |
| Feeding tube duration, days, median (range) g | 97 (3-743) | 64 (1-760) | .308 |
| Feeding tube never removed | 23 (19.5) | 18 (8.7) | .008 |
| Concomitant systemic therapy ( N = 261) | N = 93 (%) | N = 168 (%) | |
| Systemic therapy protocol | <.001 | ||
| Cisplatin every 3 wk | 30 (32.3) | 96 (57.1) | |
| Cisplatin every 1 wk | 24 (25.9) | 40 (23.8) | |
| Carboplatin ± paclitaxel | 15 (16.1) | 12 (7.1) | |
| Cetuximab | 24 (25.8) | 20 (11.9) | |
| Total cisplatin received mg/m2 , median (range) | 240 (40-300) | 240 (40-300) | .12 |
| Cumulative cisplatin > 200 mg/m2 | 43 (79.6) | 128 (94.1) | .006 |
| ST cycle delayed or cancelled | 46 (49.5) | 81 (48.2) | .949 |
| ST dose reduction in administered cycle | 5 (5.4) | 16 (9.5) | .346 |
| Switched ST protocol during RT h | 17 (18.3) | 13 (7.7) | .019 |
| Febrile neutropenia | 8 (8.6) | 5 (3) | .088 |
Data shown as N (%) unless otherwise noted.
Bold values are statistically significant values.
Abbreviations : IV, intravenous; OPC, oropharyngeal squamous cell carcinoma; RT, radiation therapy; ST, systemic therapy; TPT, treatment package time.
aDelays of 2 or more days during a completed RT course.
bTotal days elapsed between first to last fraction for a completed course of definitive RT.
cTotal days from surgery to the date of last fraction for a completed course of adjuvant RT.
dHospitalization during RT other than admission for concomitant systemic therapy or IV hydration.
eAdmission for IV hydration other than that scheduled in systemic therapy protocol.
fFeeding tube inserted prophylactically before start of RT following multidisciplinary decision.
gMedian time for feeding tubes inserted after RT start date and removed before death.
hSwitching from cisplatin every 3 wk to a more tolerable ST protocol (cisplatin weekly, carboplatin, cetuximab) due to increased toxicity.
Of the 261 patients who had concomitant ST, older patients received more cetuximab, carboplatin, and weekly cisplatin as the primary protocol ( P < .001) with a trend for more febrile neutropenia ( P =.088). Older patients were less likely than younger patients to receive cumulative cisplatin > 200 mg/m2 (79.6% vs 94.1 %; P = .006) and more likely to switch the ST protocol, from cisplatin to carboplatin, cetuximab or cisplatin weekly (after every 3 wk), (18.3% vs 7.7%; P = .019). Within older patients, except for more prophylactic feeding tube insertion before RT start in adjuvant compared to definitive CRT patients (44.4% vs 14.3%; P = .034), surgery was not associated with any added toxicity from RT or ST ( Supplementary Table 3, www.appliedradiationoncology.com ).
Multivariate Analysis: Therapeutic and Toxicity Details as Predictors of Survival
We performed MVA to assess the predictive nature of RT and ST tolerance/toxicity parameters for survival in all patients. Not completing the RT course was significantly associated with worse OS, DFS, and LRFS, with HR 5.54 to 8.12. Unplanned hospitalization (HR 1.71; 95% CI: 1.03-2.84) and RT delays (HR 2.57; 95% CI: 1.45-4.55) were predictive for OS. Feeding tubes never removed, definitive RT (vs surgery+ RT+/-ST), and diagnosis in earlier years also predicted worse outcomes ( Table 4 ).
Multivariate Analyses of Characteristics and Outcomes, Including Therapeutic Tolerance and Acute Toxicity, Associated With Survival for All Patients With Oropharyngeal Cancer Who Received Radiation Therapy With or Without Systemic Therapy
| Characteristics and Outcome | All Patients With Oropharyngeal Cancer Who Received Radiation Therapy ( N = 326) | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall Survival a | Disease-Free Survival b | Locoregional Recurrence-Free Survival c | Distant Metastasis-Free Survival d | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Marital status (married vs single) |
0.52 (0.30- 0.89) |
.018 | 0.71 (0.42- 1.21) |
.214 | ** | N/A | ** | N/A |
| Smoking pack-years (continuous) | 1.01 (1.00- 1.02) |
.028 | 1.01 (1.00- 1.02) |
.014 | 1.00 (0.98- 1.01) |
.847 | 1.01 (0.99- 1.02) |
.344 |
| N stage (N3 vs N0) | ** | N/A | ** | N/A | ** | N/A | 20.6 (2.17- 195.5) |
.008 |
| AJCC stage e | ||||||||
| 4A vs 1 | 3.85 (1.24- 11.96) |
.020 | 1.74 (0.57- 5.30) |
.328 | 0.28 (0.06- 1.35) |
.112 | ** | N/A |
| 4B vs 1 | 3.59 (1.11- 11.67) |
.033 | 1.62 (0.49- 5.37) |
.426 | 0.92 (0.18- 4.71) |
.924 | ** | N/A |
| Definitive RT (no vs yes) f | 0.35 (0.18- 0.68) |
.002 | 0.44 (0.24- 0.81) |
.009 | 0.08 (0.02- 0.32) |
<.001 | ** | N/A |
| RT delays g (yes vs no) | 2.57 (1.45- 4.55) |
.001 | 1.71 (0.99- 2.96) |
.057 | 0.95 (0.43- 2.11) |
.906 | ** | N/A |
| RT course completed (no vs yes) |
8.12 (2.84- 23.21) |
<.001 | 5.54 (2.11- 14.55) |
.001 | 7.87 (1.37- 45.3) |
.021 | ** | N/A |
| Unplanned hospitalization during RT h (yes vs no) | 1.71 (1.03- 2.84) |
.037 | 1.04 (0.63- 1.72) |
.879 | ** | N/A | 3.82 (1.57- 9.27) |
.003 |
| FT used during RT (no vs yes) |
0.93 (0.49- 1.77) |
.826 | 0.92 (0.51- 1.66) |
.776 | 0.92 (0.36- 2.37) |
.868 | 0.52 (0.2- 1.4) |
.198 |
| FT never removed (yes vs no) |
3.55 (1.89- 6.67) |
<.001 | 2.60 (1.38-4.93) | .003 | ** | N/A | 3.90 (1.56- 9.71) |
.004 |
| FT inserted before RT start i (yes vs no) | 1.74 (0.89- 3.42) |
.107 | 1.65 (0.85- 3.21) |
.139 | 3.88 (1.53- 9.83) |
.004 | ** | N/A |
| Period of diagnosis (2017-2020 vs 2009-2012) |
** | N/A | 0.58 (0.27- 1.23) |
.155 | 0.19 (0.07- 0.54) |
.002 | 0.22 (0.06- 0.77) |
.019 |
Bold values are statistically significant values; ** not included in the mutivariate analysis model
Abbreviations: AJCC, American Joint Committee on Cancer; FT, feeding tube; HPV, human papilloma virus; HR, hazard ratio; RT, radiation therapy; ST, systemic therapy.
aOverall survival adjusted for HPV status, age, total Charlson Comorbidity Index, alcohol use, oropharyngeal subsite, systemic therapy protocol, cisplatin cumulative dose and weight loss.
bDisease-free survival adjusted for HPV status, total Charlson Comorbidity Index, systemic therapy protocol and weight loss.
cLocoregional recurrence-free survival adjusted for HPV status, worst grade of pain during RT, switching systemic therapy protocol during RT and weight loss.
dDistant metastases-free survival adjusted for HPV status, T stage, systemic therapy protocol and weight loss.
eAJCC 8th version.
fDefinitive RT ± ST vs Surgery + RT+/-ST.
gUplanned RT delays of > 2 d.
hHospitalization during radiation therapy other than admission for concomitant systemic therapy or IV hydration.
iFT inserted prophylactically before RT start compared with FT inserted after RT.
Discussion
In this retrospective analysis of more than 300 patients treated curatively for OPC over 10 years, we did not observe any significant survival differences between older and younger patients, regardless of HPV status. Interestingly, overall treatment approaches for both groups were similar except for ST protocol. Both RT and ST were equivalently well tolerated in older and younger patients, although significantly higher proportions of older patients had unplanned hospitalizations and feeding tube insertions, and they were more likely to require switching to a less intense ST protocol. Thus, we emphasize that more multidisciplinary supportive care may be required for older patients, especially when concomitant ST is used with RT. Importantly, we explored specific RT and ST details, revealing treatment tolerability and toxicity across age groups, shedding light on the clinical consequences of contemporary strategies for treating OPC in an aging population.
In the years comprising our study period, we observed a steady increase in the number of older patients with OPC and a significant increase in the number of patients with HPV+ve OPC, which aligns with other findings.4, 6, 9, 12 Xu et al projected that by 2025, 32.6% of all cases of OPC will occur in patients > 70 years, which approximates the 38% that we saw in our older cohort aged ≥ 65 years between 2017 and 2020.33
While most previous studies have focused mainly on OS in patients with HPV+ve OPC, our analysis is unique because we evaluated tumor-specific survival endpoints and considered both HPV+ve and HPV-ve status in combination with age. Similar to our findings, a Surveillance, Epidemiology, and End Results (SEER) database study of 355 patients observed no difference in receipt of treatment or 3-year OS in patients with HPV+ve OPC across age groups. Nevertheless, the SEER study did not assess other tumor-specific endpoints, and it included metastatic patients.34 Dickstein et al evaluated a cohort of 88 older patients with OPC and observed similar survival rates as those in our study; however, they did not include a younger patient comparison group.13 In contrast to the findings of a National Cancer Database subgroup analysis of 21,880 patients with HPV+ve OPC, we did not see any differences in disease stage or receipt of surgery in older patients.9 In a post hoc analysis of patients with locally advanced OPC treated with definitive RT in the RTOG-0129 and RTOG-0522 randomized trials, older age defined as ≥ 50 years was independently prognostic for DMFS, which was not seen in our analysis, although advanced cancer stage and smoking history were predictive of worse outcomes, similar to our findings. Importantly, that analysis included all-cause mortality in addition to distant metastatic events within the definition of DMFS, unlike our definition that we believe is more clinically relevant.20 Lastly, in agreement with our results, Windon et al showed a nonsignificant difference in OS between age groups for patients with HPV-ve OPC, although no other endpoints were studied, and no multivariate analysis was performed.10 Overall, although study designs have varied greatly, a clearer picture of the important features associated with different survival endpoints in older patients with OPC is emerging.
One strength of our study is that we analyzed 91 patients (27%) who had undergone surgery, mainly TORS. In our practice, age is not a major factor for precluding surgery since excellent outcomes have been shown with TORS in older patients,25 and that is why surgery rates were nondifferent with age. Including patients who had surgery gave us the opportunity to compare pathological risk factors by age in this group, and we did not note any distinct features for older patients. Nevertheless, rates of perineural invasion, lymphovascular space invasion (LVSI), and ENE in our older cohort were different from other studies.12, 13 But similar to our findings, a recent study of 136 patients with HPV+ve OPC who underwent TORS showed that age ≥ 65 years was not associated with ENE, positive final surgical margin, perineural invasion, or LVSI.35 We did not note significantly increased toxicity by surgery for older patients who received adjuvant ( n = 9) compared with definitive CRT ( n = 84), albeit limited number obviates solid conclusions. Thus, more targeted studies of how older patients respond to surgery in combination with RT and ST are needed.
Considering the equivalent survival and good prognoses for older patients observed across numerous studies, especially in patients with HPV+ve OPC, more focus is needed on exploring treatment tolerance and toxicity to leverage the overall therapeutic index. More than half of our older patients received concomitant cisplatin, which is more than the 33% reported by Dickstein et al, and we noted more hospitalizations, grade 3-4 mucositis, and treatment interruptions in our older cohort, although we observed comparable survival rates.13 Strom et al reported more hospitalizations, PEG tube dependence, more frequent use of concomitant cetuximab and carboplatin in older than in younger patients, in agreement with our analysis. However, we did not see more dose reductions.36 In contrast to other studies, we did not observe more RT interruptions in older patients.37
Notably, our study is only one of a few that have reported toxicity rates from RT and ST specifically for patients with OPC, unlike most studies that have encompassed all HNCs. We noted higher rates of feeding tube use, both transient and for life, in the older group, which was also shown by Sachdev et al, who concluded that older age was the only predictor for feeding tube use.38 Of note, in addition to being associated with OS in other studies,39 never removing a feeding tube was also predictive of dismal DMFS in our study. In agreement with our findings, Mayer et al observed that mucositis, weight loss, and leukopenia/infection did not differ with age in a matched-pair analysis of older vs younger patients receiving adjuvant chemoradiotherapy.40 Although we did not see differences based on age, we observed that both components of RT interruptions (incomplete and delayed RT) were independently detrimental for OS, as reported by others.40, 41 Therefore, our analysis emphasizes that starting with an optimal ST protocol in older patients is advocated as long as treatment providers are flexible and able to reduce doses or switch to a less intense ST protocol once intolerable toxicities are encountered.
With a rising number of older adults being diagnosed with OPC, prospective randomized studies targeting this population are being conducted. Although not exclusive to OPC, the ongoing EGeSOR (NCT02025062), ELAN-FIT (NCT01864772), ELAN-UNFIT (NCT01884623), and ELAN-RT (NCT018648350) trials are dedicated to investigating older adults. The ELAN geriatric evaluation, which takes only 20 minutes to determine patient eligibility for any of the 3 ELAN studies, has been validated and represents an important tool for studying older populations.42 The final results of these trials will shed light on the utility of a robust stratification tool for objectively evaluating the fitness of older patients for curative therapies, thereby leveraging survival and QOL outcomes. Nevertheless, recent de-escalation studies for patients with HPV+ve OPC have not stratified for age, and few older patients participated, even though de-escalation is a very desirable option for older groups.43 - 46
Our study had several limitations, including the lack of a formal baseline and follow-up QOL measurement, and no formal geriatric assessment (GA) tool for older patients. The clinical integration of GA remains limited in current practice for OPC treatment, highlighting a gap in providing comprehensive, personalized care to older adults with OPC. Partially relevant to GA, we observed that baseline CCI was predictive for worse OS in older patients, with no impact on tumor-specific outcomes that was consistent with others.28
We were also not able to capture all chemotherapy-related toxicities as in other studies,13, 36, 40 although we believe that febrile neutropenia, unplanned hospitalizations, and dose modifications are all objective surrogate measures for serious toxicity. We did not compare late toxicities/morbidities such as pneumonia and malnutrition that have been associated with increased risk of death.45 Of note, we were not able to compare different ST protocols among older patients regarding toxicity. Nevertheless, this comparison is hard, taking into consideration the limited number of patients and the high rates of delayed and cancelled cycles (48%) and the rate of switching to less intense protocols (18%). However, despite having less intense protocols and less cumulative cisplatin doses in the older group, they suffered higher toxicities and more hospitalizations. We observed higher than usual feeding tube use in older patients, partially because temporary nasogastric tubes were included with PEG tubes. Importantly, the definition and survival implications for patients with “feeding tube never removed” should be interpreted with caution because it included patients who died in the early post-treatment interval with recurrence before removing the tube, while it is not unusual for some fit patients to have feeding tube dependency up to 2 years following RT. TPT was similar with age, suggesting no significant delays due to postoperative complications for older patients managed with surgery. We included only curatively treated patients in our analyses, which may have resulted in selection bias or the confounding effect by indication against older unfit patients with poor overall health status and functional abilities, which might have skewed survival outcomes. Older patients with OPC often present with diverse comorbidities and varying levels of frailty, potentially influencing treatment decisions and outcomes. Limited numbers of patients did not permit a proper matched comparison for age groups based on stage, OPC subsite, treatment category, or ST protocol.
Conclusions
For patients with OPC, older age ≥ 65 years was not associated with any unique clinicopathological characteristics, specific treatment strategies, or worse survival outcomes, regardless of HPV status. Although older patients experienced higher levels of therapeutic toxicity and feeding tube insertions, they did not experience more RT interruptions than younger patients. Our findings underscore the complexities of treatment tolerance and toxicity profiles in this population, often underrepresented in clinical trials. By evaluating functional status, comorbidities, and geriatric syndromes, GA can guide tailored treatment strategies that optimize efficacy outcomes while minimizing treatment-related morbidity in older patients.
Until results from ongoing de-escalation and immunotherapy studies are available, every effort should be made to deliver optimal SoC therapy to patients with OPC regardless of age. Trials dedicated to investigating older patients with OPC are needed to prospectively address treatment tolerability and the key factors affecting QOL in addition to survival.
References
Citation
Ahmed I. Ghanem, MD, PhD, Marissa Gilbert, BS, Chun-Hui Lin, MS, Remonda Khalil-Moawad, MPH, Samantha Tam, MD, Steven Chang, MD, FACS, Farzan Siddiqui, MD, PhD. Disease Characteristics, Treatment Tolerance, and Toxicity in Older Patients (≥ 65 y) With Oropharyngeal Cancer and Implications on Outcomes. Appl Radiat Oncol. 2024;(4):1 - 15.
doi:10.37549/ARO-D-24-00027
December 1, 2024