Electronic brachytherapy for skin cancer: Problems and progress
Images
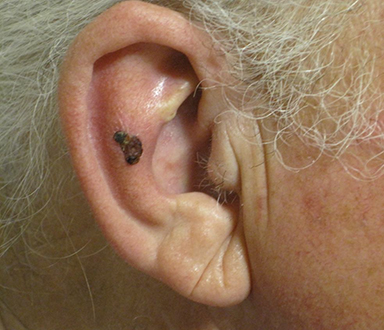
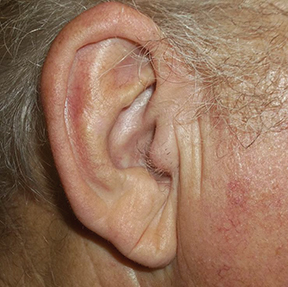
Over the last several years, the growing use of electronic brachytherapy (EBT) for nonmelanoma skin cancer (NMSC) has met with concerns, including physician self-referral and its use in the Medicare population. While evidence may show EBT as having high long-term curative rates with minimal side effects from local radiation, many—such as authors of a 2015 viewpoint article in JAMA Dermatology—say adequate data has yet to be accumulated.1
One author of the article, Jack Resneck, Jr., MD, professor and vice chair of dermatology at the University of California, San Francisco, said that the “appropriate solution is probably to have new CPT codes that will be valued appropriately for skin brachytherapy.”2 And, in January 2016, new category III codes from the American Medical Association for the treatment of skin cancer took effect. For HDR EBT, skin surface application, per fraction, including basic dosimetry, the code is 0394T.
In November 2015, an article in Medical Devices: Evidence and Research discussed EBT as a novel treatment option for NMSC and described 3 EBT systems: Xoft Axxent (iCAD, Inc.; Nashua, New Hampshire), Intrabeam (Carl Zeiss Meditec; Dublin California), and the Esteya (Elekta; Stockholm, Sweden).3 In their discussion, the authors suggest that “radiotherapy for NMSC is likely underutilized” and conclude that, “EBT appears to be a quick and convenient method to replicate, and possibly improve upon, other radiotherapy techniques for small, superficial lesions.”
While the American Academy of Dermatology’s 2014 position statement on EBT for NMSC supports consideration of EBT as a secondary option for basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) in special circumstances when surgical intervention is contraindicated or the patient refuses surgical management, it states that surgical management is the most effective treatment for BCC and SCC. The statement also calls for additional long-term outcomes research on EBT use.
Recent Clinical Studies
While no long-term outcomes data currently support the efficacy of EBT, a handful of single-center, short-term outcomes data show promise.
Gauden et al assessed Leipzig surface applicators (Varian Medical Systems, Palo Alto, California) for high-dose rate (HDR) brachytherapy for treating NMSC. In 200 patients with 236 lesions, 36 Gy was given in daily 3 Gy fractions in an area between 3-4 mm. Of the lesions, 121 were BCCs and 115 were SCCs. Local control was 98%, and grade 1 skin toxicity was detected in 71% of the lesions while grade 2 was detected in 34%. Overall, the authors reported good to excellent cosmesis in 85% of the patients (208 cases), with late skin hypopigmentation changes observed in 5.5% (13 cases).4
Another single-center study reported good to excellent cosmesis, acceptable toxicities at 1 year and no recurrences at 1 year. While the study included 122 patients with 171 nonmelanoma lesions treated with EBT, 40 Gy in 8 fractions delivered twice weekly, follow-up data at 1 year or more was available in 42 patients with 46 lesions. No grade 3 or higher adverse events were reported during the study or follow-up. Cosmesis at 1 year was excellent in 92.9% and good in 7.1% of the lesions.5
In a retrospective analysis of 127 patients with 154 NMSC lesions treated with HDR EBT, 40 Gy in 8 fractions, authors evaluated local control, acute toxicity, late toxicity and cosmetic outcomes. Grade 0-1 acute radiation dermatitis was detected in 52.6%, grade 2 in 34.4% and grade 3 in 13% of the treated lesions. Late toxicity, grade 0-1, was observed in 94.2% and grade 2 in 5.8% of all cases. Cosmesis was excellent in 94.2%, good in 3.3%, fair in 0.7% and poor in 0.7% of treated lesions. The authors conclude that HDR EBT should be considered ideal for NMSC of the head, neck and central facial locations where surgical cosmesis may be inferior.6
Using the Valencia skin applicator (Elekta, Stockholm, Sweden), 32 patients with 45 NMSC lesions received a dose of 42 Gy in 6 or 7 fractions with a depth of 3 mm delivered twice each week. The authors reported 98% local control at 47 months post-treatment, and only grade 1 skin toxicity that was resolved with topical treatment. According to the authors, superficial BCC lesions < 25 mm in maximum diameter treated with the Valencia applicator using a hypofractionated treatment offers excellent results for cosmesis and local control at 3-year follow-up.7
Another retrospective case series of 57 lesions in 39 elderly (> 70 years), eligible patients were treated with HDR brachytherapy using the Valencia surface applicator. A prescribed dose of 40 Gy in 8 fractions was used to treat 48 lesions, and 50 Gy in 10 fractions was used in 9 lesions; all treatments were delivered 2-3 times a week. At 12 months, 96.25% of the lesions demonstrated a complete response while 2 cases had a partial remission. Overall, cosmesis was excellent in 86%, good in 10.5% and fair in 2.3% of the lesions.8
Clinical Implementation
In a 2014 review paper, clinicians discussed implementation of the Esteya system in their facilities.9
The authors followed The American Joint Committee on Cancer (AJCC) criteria for inclusion of patients with clinical stage T1 or T2 status. The maximum diameter was 20 mm with a maximum depth of 3-4 mm measured by ultrasound or punch biopsy.
A prescription depth of 3 mm was determined for lesions with a depth of < 3 mm while deeper lesions had a maximum depth of 5 mm. Based on recent data supporting a 2 mm dermoscopically detected excision margin that achieved histologically confirmed complete excisions in 98.5% of cases,10 the authors utilized a dermatoscope to assess gross tumor volume (GTV).
The authors determined a biologically equivalent dose (BED) of around 70 Gy with an alpha/beta value of 10; the selected dose prescription was 42 Gy in 6 fractions (7 G/fraction) twice each week for a total BED of 71.4. A quality assurance check was performed each day before treatment.
The authors found that the Esteya system was simple for both providers and patients, allowing for safe, precise treatment of NMSC.
Conclusion
While there is unquestionably a need for continued research examining patient outcomes after treatment with EBT for NMSC, early data looks promising in terms of cosmesis, toxicity and short-term response. While Mohs surgery will remain the standard of care for many NMSC patients, EBT provides options in cases where surgery is not a viable, or patient-preferred, therapy.
References
- Linos E, VanBeek M, Resneck JS. A sudden and concerning increase in the use of electronic brachytherapy for skin cancer. JAMA Dermatol. 2015;151(7):699-700.
- Castellino AM. Concerns over sudden increase in radiation for skin cancer. Medscape, October 21, 2015. http://www.medscape.com/viewarticle/852921. Accessed May 17, 2017.
- Kasper ME, Chaudhary AA. Novel treatment options for nonmelanoma skin cancer: focus on electronic brachytherapy. Med Devices (Auckl). 2015;8:493-502.
- Gauden R, Pracy M, Avery AM, et al. HDR brachytherapy for superficial non-melanoma skin cancers. J Med Imaging Radiat Oncol. 2013;57(2):212-217.
- Bhatnagar A. Nonmelanoma skin cancer treated with electronic brachytherapy: Results at 1 year. Brachytherapy. Mar-Apr 2013;12(2):134-140.
- Paravati AJ, Hawkins PG, Martin AN, et al. Clinical and cosmetic outcomes in patients treated with high-dose-rate electronic brachytherapy for nonmelanoma skin cancer. Pract Radiat Oncol. 2015;5(6):e659-664.
- Tormo A, Celada F, Rodriguez S, et al. Non-melanoma skin cancer treated with HDR Valencia applicator: clinical outcomes. J Contemp Brachytherapy. 2014;6(2):167-172.
- Delishaj D, Laliscia C, Manfredi B, et al. Non-melanoma skin cancer treated with high-dose-rate brachytherapy and Valencia applicator in elderly patients: a retrospective case series. J Contemp Brachytherapy. 2015;7(6):437-444.
- Pons-Llanas O, Ballester-Sanchez R, Celada-Alvarez FJ, et al. Clinical implementation of a new electronic brachytherapy system for skin brachytherapy. J Contemp Brachytherapy. 2014;6(4):417-423.
- Caresana G, Giardini R. Dermoscopy-guided surgery in basal cell carcinoma. J Eur Acad Dermatol Venereol. 2010;24:1395-1399.
Citation
MB M. Electronic brachytherapy for skin cancer: Problems and progress. Appl Radiat Oncol. 2017;(2):24-25.
June 2, 2017