Examining risk factors for rectal toxicity following radiation therapy for localized prostate cancer
Images
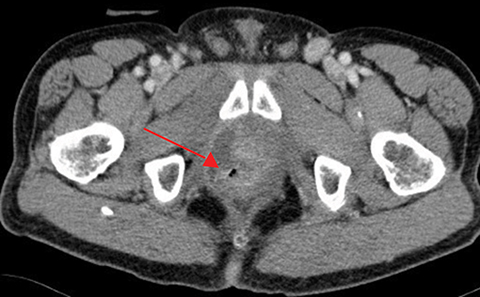
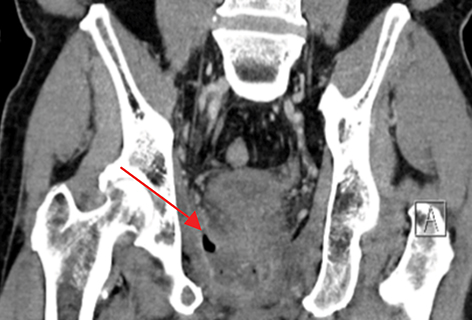
Radiation-induced bowel toxicity, such as radiation proctitis, is a relatively common side effect following radiation therapy (RT) for prostate cancer. Risk factors for late radiation bowel toxicity include patient-related factors such as smoking, hypertension, diabetes and atherosclerosis.1 Treatment-related factors include the presence of seminal vesicle and/or pelvic irradiation, RT technique and total dose, as well as specific rectal dose-volume parameters such as the V30 and V60.2-4
These toxicities are generally graded on a scale based on symptom severity. The RTOG (Radiation Therapy Oncology Group) classification describes the severity of acute gastrointestinal toxicity, whereas the RTOG/EORTC (European Organization for Research and Treatment of Cancer) scoring system categorizes severity of chronic gastrointestinal toxicity.5 The vast majority of bowel toxicity (> 90%) is grade 1 or 2.6 However, approximately 5% of patients will experience higher grade toxicities, which are often refractory to initial treatment strategies and require more aggressive management.7
The aim of this case report is to examine potential, and possibly novel, risk factors that may have contributed to the development of severe rectal toxicity in a patient treated with external-beam RT for localized prostate cancer.
CASE SUMMARY
We present the case of a 70-year-old Haitian man whose past medical history is remarkable for type II diabetes mellitus, essential hypertension, hypercholesterolemia, hemorrhoids, an ischemic stroke with no lasting sequelae, and a coronary angioplasty in 2006. The patient was investigated for prostate cancer following a rise in his prostate specific antigen (PSA) over several years. An ultrasound-guided biopsy was performed in 2014 and confirmed the presence of Gleason score 7 (3 + 4) prostate adenocarcinoma on all 12 biopsies as well as a small periprostatic foci of Gleason score 8 (4 + 4) indicating extra-prostatic invasion. The clinical stage was T2c and the PSA was 15. His International Prostate Symptom Score (IPSS) was 2 at the initial consultation. Given the patient’s high-intermediate risk disease, the diagnostic workup was expanded to include a bone scan and pelvic CT, all of which were negative for metastases.
The patient was started on monthly degarelix acetate subcutaneous injections and then received external-beam volumetric modulated arc therapy (VMAT) within 2 weeks. He was treated to a total dose of 78 Gy in 39 fractions that included pelvic nodal irradiation (44 Gy). Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) dose constraints were respected.4
After 10 fractions of RT, the patient developed a region of moist desquamation in the intergluteal cleft. He was prescribed silver sulfadiazine cream, offering little improvement. He was referred to a dermatologist who performed a punch biopsy of the lesion. Biopsy confirmed a herpetic lesion and the patient was given oral valacyclovir. The rest of the RT course was unremarkable. Serial PSA measurements at 2, 4, 6 and 8 months after the end of RT were 2.76, 1.39, 0.71, 0.47, respectively. After 6 months of degarelix acetate, the patient refused to pursue treatment due to the sexual toxicity.
The patient did not experience any late genitourinary side effects; however, his hemorrhoidal disease worsened, requiring a hemorrhoidectomy approximately 2 months after completing RT. Following surgery, the patient’s lower digestive symptoms resolved temporarily. Unfortunately, he developed recurrent anorectal pain 6 months after completing RT (4 months after hemorrhoidectomy). A subsequent colonoscopy was performed and showed a rectal lesion suspicious of a primary rectal neoplasm. A biopsy of the lesion was performed and demonstrated a radiation-induced rectal ulcer. Initial medical therapy, including sulcralfate enemas, was ineffective.
The patient was referred and received 40 sessions of hyperbaric oxygen therapy with little to no symptomatic improvement. The patient was hospitalized to optimize his pain medication and perform a radiologic workup. CT imaging of the abdomen and pelvis revealed a perirectal abscess that required a diverting colostomy and intravenous antibiotics, as well as a recto-urethral fistula. Follow-up CT imaging at 1, 2 and 3 months after surgery and antibiotics showed no improvement in the abscess with fistulization in the levator ani muscle (Figure 1). Further surgical management of the abscess and fistula was assessed. Due to the extensive surgery that would be required and the high risk of complications, the consensus was to follow the patient with serial imaging and optimize his pain control. Currently, 8 months after the treatment with hyperbaric oxygen, the patient is followed by pain medicine specialists and requires opioids including methadone for pain management. His PSA at last follow up in May 2016 was 0.5 ng/ml.
DISCUSSION
In examining this case, several factors likely to contributed to the patient’s overall toxicity and clinical course. First, the patient’s multiple comorbidities including type II diabetes, atherosclerotic heart disease and hypertension are all vascular risk factors that likely increased the probability of radiation toxicity. Also, as demonstrated by several published nomograms, the use of anticoagulants, presence of hemorrhoids
and use of androgen deprivation can contribute to increased lower GI toxicity.4
Second, the multiple biopsies and hemorrhoidectomy may have increased the patient’s risk of abscess formation or fistulization. A recent review concluded that rectal biopsies may initiate chronic wounds or infections, do not contribute to the diagnosis of chronic radiation proctitis and, thus, should be avoided unless deemed necessary to eliminate suspicion of a neoplastic lesion.8 Other studies have described fistula formation following rectal biopsies.9-11 Interestingly, in a study by Chrouser et al, 38% of patients who developed rectal fistulas after RT had undergone a prior rectal biopsy.11 This supports the hypothesis that in an irradiated field, further tissue damage from interventions such as a biopsy, likely increase the risk of fistula and/or abscess formation. With regard to the hemorrhoidal surgery, due to the much more proximal localization of the rectal ulcer in relation to the site of surgery, it is unlikely this intervention contributed to the development of the rectal ulcer.
Another consideration is whether the use of a high-dose-rate brachytherapy (HDR) boost may have produced a different outcome in this patient. Given that this patient’s dosimetry was well within acceptable limits, there was no formal indication to favor an HDR boost over VMAT alone for this patient. However, in our experience, the use of a single-fraction HDR boost can often limit the V75 (volume of rectum receiving 75% of the prescription dose) to 1 to 2 cc since no PTV is used. In contrast, this hypofractionated technique uses a larger dose per fraction (often 15 Gy in a single fraction) and may potentially have opposite repercussions on normal tissues. Using an alpha/beta = 3 Gy, the EQD2 for an HDR boost of 15 Gy in 1 fraction is 54 Gy. To our knowledge, it is unknown what impact achieving a lower volume of irradiated rectum, and using a high dose per fraction, would have on long-term rectal toxicity. As such, it is unclear what impact an HDR boost would have had in our patient.
One may question whether the use of hyperbaric oxygen therapy (HOT) was indicated for our patient or if it may have led to increased bacterial proliferation in a patient already at risk of infection following a rectal biopsy. HOT involves patients breathing pure oxygen in a pressurized room or tube at 3 times the normal air pressure.12 These conditions lead to highly oxygenated blood, which may be beneficial because it inhibits bacterial growth and stimulates the release of growth factors and stem cells, promoting wound healing and possibly reversing progressive changes caused by RT.13,14 HOT is generally recommended in cases of radiation proctitis after initial medical pharmacotherapy has failed. A Cochrane review revealed a significantly increased chance of improvement or cure following HOT for radiation proctitis (RR 1.72; 95% CI 1.0 to 2.9, p = 0.04).15 Therefore, it does not appear likely that our patient’s HOT contributed to further GI toxicity. The ideal HOT regimen is not known; however, one randomized trial used 30 daily sessions with an option for additional sessions if a clinical improvement was noted.16
Also, we have considered whether our patient’s oral antiviral therapy may have played a role in increasing his risk of GI toxicity. Published animal and human phase I-II clinical trials have investigated the potential therapeutic effect of adenovirus mediated gene therapy combined with RT for localized prostate cancer.17-20 This treatment approach often involves intraprostatic insertion of either an adenovirus gene vector followed by subsequent administration of an antiviral prodrug such as valacyclovir. RT was initiated 48 hours after the start of antiviral therapy. Gene therapy was not associated with any grade 3 or higher toxicity and, at 5 years, no late side effects were reported.21 Despite these results, it remains unclear whether antiviral therapy in patients with viral lesions in noncancerous tissues may act as a radiosensitizer and increase RT toxicity.
Finally, it is well-known that the toxicity profile patients experience for a given dose of RT varies considerably, depending on differences in underlying individual normal tissue radiosensitivity.22 Several rare genetic syndromes such as ataxia telangiectasia and Nijmegen syndrome that are characterized by mutations in genes in the detection and repair of DNA damage are associated with accrued sensitivity to ionizing radiation.23,24
Currently, the investigation of potential genetic differences to explain variable radiation sensitivity is an area of intense research. Genome-wide association studies (GWAS) have revealed polymorphisms associated with radiation toxicity risk.25,26 The possibility of a genetic predictive risk “signature” is, therefore, promising. As many patient and treatment-related factors affect the overall risk of toxicity for a given dose, new risk models need to be developed that combine patient, treatment and genetic data.
CONCLUSION
In summary, our patient’s clinical course represents a rather exceptional case of the development of multiple late radiation toxicities. Although this patient’s comorbidities placed him at higher risk of developing radiation-related toxicities, other factors were also likely to be involved. Rectal biopsies are rarely indicated and should be avoided in the setting of GI radiation injury as they may facilitate further complications, as was the case for our patient.
REFERENCES
- Grodsky MB, Sidani SM. Radiation proctopathy. Clin Colon Rectal Surg. 2015;28(2):103-111.
- Chan LW, Xia P, Gottschalk AR, et al. Proposed rectal dose constraints for patients undergoing definitive whole pelvic radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72(1):69-77.
- Michalski JM, Gay H, Jackson A, et al. Radiation dose–volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76(3):S123-S129.
- Valdagni R, Rancati T, Fiorino C, et al. Development of a set of nomograms to predict acute lower gastrointestinal toxicity for prostate cancer 3D-CRT. Int J Radiat Oncol Biol Phys. 2008;71(4):1065-1073.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
- Roach M. Reducing the toxicity associated with the use of radiotherapy in men with localized prostate cancer. Urol Clin North Am. 2004;31(2):353-366.
- Hayne D, Vaizey CJ, Boulos PB. Anorectal injury following pelvic radiotherapy. Br J Surg. 2001;88(8):1037-1048.
- Vanneste BG, Van De Voorde L, de Ridder RJ, et al. Chronic radiation proctitis: tricks to prevent and treat. Int J Colorectal Dis. 201530(10):1293-1303.
- Thornhill J, Long RM, Neary P, et al. The pitfalls of treating anorectal conditions after radiotherapy for prostate cancer. Ir Med J. 2012.
- Theodorescu D, Gillenwater JY, Koutrouvelis PG. Prostatourethral‐rectal fistula after prostate brachytherapy. Cancer. 2000;89(10):2085-2091.
- Chrouser KL, Leibovich BC, Sweat SD, et al. Urinary fistulas following external radiation or permanent brachytherapy for the treatment of prostate cancer. J Urol. 2005;173(6):1953-1957.
- Feldmeier JJ, Hampson N. A systematic review of the literature reporting the application of hyperbaric oxygen prevention and treatment of delayed radiation injuries: an evidence based approach. Undersea Hyperbaric Med. 2002;29(1):4-30.
- Marx RE, Ehler WJ, Tayapongsak P, Pierce LW. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am J Surg. 1990;160:519-524.
- Marx RE. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg. 1983; 41(6):351-357.
- Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016; Apr 28;4:CD005005.
- Clarke RE, Tenorio LM, Hussey JR, et al. Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomized and controlled double-blind crossover trial with long-term follow-up. Int J Radiat Oncol Biol Phys. 2008;72(1):134-143.e15.
- Teh BS, Ayala G, Aguilar L, et al. Phase I–II trial evaluating combined intensity-modulated radiotherapy and in situ gene therapy with or without hormonal therapy in treatment of prostate cancer–interim report on PSA response and biopsy data. Int J Radiat Oncol Biol Phys. 2004;58(5):1520-1529.
- Freytag SO, Stricker H, Pegg J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate-to high-risk prostate cancer. Cancer Res. 2003;63(21):7497-7506.
- Freytag SO, Khil M, Stricker H, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002;62(17):4968-4976.
- Freytag SO, Movsas B, Aref I, et al. Phase I trial of replication-competent adenovirus-mediated suicide gene therapy combined with IMRT for prostate cancer. Mol Ther. 2007;15(5):1016-1023.
- Freytag SO, Stricker H, Peabody J, et al. Five-year follow-up of trial of replication-competent adenovirus-mediated suicide gene therapy for treatment of prostate cancer. Mol Ther. 2007;15(3):636-642.
- Barnett GC, Kerns SL, Noble DJ, et al. Incorporating genetic biomarkers into predictive models of normal tissue toxicity. Clin Oncol. 2015;27(10):579-587.
- Little JB, Nove J. Sensitivity of human diploid fibroblast cell strains from various genetic disorders to acute and protracted radiation exposure. Radiat Res. 1990;123(1):87-92.
- Taylor A, Harnden DG, Arlett CF, et al. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature. 1975;258(5534):427-429.
- Barnett GC, Thompson D, Fachal L, et al. A genome wide association study (GWAS) providing evidence of an association between common genetic variants and late radiotherapy toxicity. Radiother Oncol. 2014:111(2):178-185.
- Kerns SL, West CM, Andreassen CN, et al. Radiogenomics: the search for genetic predictors of radiotherapy response. Future Oncol. 2014;10(15):2391-2406.
Citation
D T, D T, C L. Examining risk factors for rectal toxicity following radiation therapy for localized prostate cancer. Appl Radiat Oncol. 2016;(4):45-47.
December 12, 2016