FLASH Radiation Therapy: Review of the Literature and Considerations for Future Research and Proton Therapy FLASH Trials
Images
Radiation therapy (RT) is routinely used in cancer care but may cause acute- and long-term toxicities as a consequence of ionizing radiation deposition in normal tissues surrounding cancer cells. These potentials for toxicities can often limit the dose of RT that can be delivered safely in the curative setting. Additionally, the risks of toxicities are often amplified with the delivery of concurrent chemotherapy or when RT is delivered as part of multimodality treatment.1
One method being increasingly employed to reduce acute- and long-term side effects commonly encountered with traditional photon therapy is the use of proton therapy. Mechanistically, protons are heavier, charged particles exhibiting unique physical properties compared with photons or electrons more traditionally used for RT.2 Protons can be delivered with precise energies to a desired depth, preferentially depositing energy at a specific depth known as the Bragg Peak, and have no exit dose. Photons, on the other hand, experience an exponential attenuation with increasing depth beyond the first few centimeters of entrance and peak dose, and they continue to deposit their energy in normal tissues beyond the tumor, thus exposing and potentially damaging normal tissue distal to the target volume.3 These spatial advantages of proton therapy dose distribution have demonstrated improved clinical outcomes and reduced toxicities for subsets of patients with head and neck cancers,4 esophageal cancers,5 lung cancers,6 liver cancers,7 pediatric malignancies,8 and others, as well as to better preserve performance status9 and quality of life10 across multiple disease sites. Additionally, proton therapy in select cases may be a safer way to deliver dose escalation and/or hypofractionation11,12 and reirradiation.13
The intrinsic spatial advantages of charged particle RT have been explored in depth, yet the effects of dose rate on the therapeutic index have only recently received increased attention. Indeed, the use of ultrahigh dose rate “FLASH” proton RT holds the potential to further reduce toxicities and to be a transformative advancement in the field of radiation oncology. Initial preclinical in vitro and in vivo studies have shown that when RT is delivered at dose rates that far exceed those currently used in routine clinical practice, fewer toxic effects of RT are exhibited. This normal tissue protection at ultrahigh dose rates is termed the FLASH effect.14 FLASH effects are thought to require dose rates delivered in excess of 40 Gy per second, whereas linear accelerators and proton accelerators used in clinical practice conventionally deliver dose at 0.06 to 0.40 Gy/s and 1.67 Gy/s, respectively. Furthermore, recent preclinical studies have suggested that such ultrahigh dose rates maintain treatment efficacy while decreasing the likelihood of toxicities.15,16
Research on FLASH RT is still in its infancy; future studies will be critical to verify whether FLASH RT will be a paradigm-changing innovation in the RT field or one of no true clinical benefit. In this manuscript, we review the proposed mechanisms of action for FLASH RT, summarize early preclinical results, discuss the first-in-human treatments with a focus on proton FLASH, and highlight challenges and future considerations of FLASH RT.
Mechanism of Action
The mechanism of action for FLASH RT’s reduced toxicity is postulated to be multifactorial. FLASH RT can produce oxygen depletion that mimics hypoxia in normal tissue. A lack of oxygen in normal tissue prevents free radicals from reacting with oxygen to form damaging peroxyl radicals. This effect results in the subsequent increase in normal tissue radioresistance,15,16 but the mechanisms by which RT-induced hypoxia might lead to differential effects between normal tissue and tumor tissue radiosensitivity remain controversial.17 In addition to, or possibly in concert with, differential reactive oxygen species production, FLASH RT may alter the DNA damage response. Indeed, conventional RT induces G2 arrest and, therefore, radiation-induced apoptosis.18 In one investigation, G2 cell cycle arrest was found to be significantly less pronounced 10 hours after irradiation with FLASH RT compared with conventional RT, which may allow for less normal tissue damage.19 Other investigators have found that the yield of DNA double strand breaks as measured by γ-H2AX foci formation is less with FLASH than conventional RT,17,20 possibly leading to a differential inflammatory response.
In this regard, FLASH RT may also induce differential expression of transforming growth factor beta (TGF-β), which is a pro-inflammatory cytokine. In one investigation, when measured 24 hours post-RT, FLASH RT only led to a 1.8 times increase in TGF-β levels, whereas conventional RT resulted in a 6.5-fold increase.20 As a result, the amount of radiation-induced chronic inflammation and fibrosis may be less with FLASH RT relative to conventional-dose rate RT.21-23
Finally, FLASH RT has been associated with greater preservation of stem cells in normal tissue relative to conventional RT. In studies of acute intestinal damage following 15 Gy whole abdominal RT, mice treated with FLASH RT showed a significantly higher number of proliferating crypt stem cells compared with mice receiving conventional-dose rate RT.23 In another study, while both conventional-dose rate RT and FLASH RT were found to be toxic for normal human hematopoiesis cells as reported by Chabi et al, only FLASH RT led to the preservation of stem cells.24 Notably, hematopoietic stem cells exist in a lower oxygen environment than the circulating blood cells,25 which could theoretically enhance the ability of FLASH-mediated depletion of molecular oxygen to achieve radiobiologically protective levels of hypoxia.
Preclinical Studies
In laboratory studies, electron FLASH RT led to fewer toxicities compared with conventional-dose rate RT. Favaudon et al irradiated C57BL/6J mice with conventional (0.03 Gy/s) or FLASH RT (≥40 Gy/s), observing significant fibrosis in the former and no apparent damage in the latter, akin to normal tissue without any irradiation.22 In tissue samples of 6 cats with locally advanced T2/T3N0M0 squamous cell carcinoma of the nasal planum treated in a single-dose escalation trial with FLASH RT (25-41 Gy), FLASH RT led to observations of no erythema, no moist desquamation, no fibronecrosis, no hyperkeratosis, no inflammatory infiltrates and no dermal remodeling.26 At 3 and 6 months, all cats experienced a complete response. One cat experienced clinical recurrence at 8 months and was euthanized shortly thereafter; the remaining 5 cats all had complete responses at 16 months. Across multiple studies, FLASH RT generally has been reported to provide better normal tissue protection with a dose modifying factor of 1.4 to 1.8.22-23,26-28
While FLASH RT has been shown to spare normal tissues, reported preclinical studies to date do not suggest that it protects tumors. Tumor kill has consistently been similar with – and in some reports, potentially even improved following – FLASH RT relative to conventional-dose rate RT. The observed dose rate-response relationship, in which higher dose rates may be associated with better tumor killing than standard dose rates, has been observed in conventional RT. Lohse et al29 compared high dose per pulse flattening filter-free beam with standard flattened beam. They reported the most efficient tumor killing at the higher dose of 0.4 Gy/s, compared to 0.066 Gy/s or 0.003 Gy/s.
First-in-Human Case Study
The first patient reported to be treated with FLASH RT was a 75-year-old man in Switzerland, diagnosed with CD30+ T-cell lymphoma and classified as T3N0M0B0.30 No prior treatments (corticoids, PUVA-therapy, Neotigason, Caryolisin, Methotrexate, Targretin, histone deacetylase inhibitor, Caelyx, brentuximab, resminostat) were successful at controlling this patient’s disease. He had been treated with RT at 110 tumor sites, most frequently administered to 20 Gy in 10 fractions or 21 Gy in 6 fractions, but the patient continued to experience various ulcerative and/or painful cutaneous lesions. FLASH-RT was administered to him with the hypothesis that it could provide equivalent tumor control while also incurring fewer skin toxicities in this heavily pretreated patient.
A 3.5-cm diameter skin tumor was treated with 15 Gy delivered over 90 milliseconds, equivalent to 167 Gy/s. Tumor shrinkage began 10 days after irradiation, and a complete response was noted at 36 days; tumor response was durable for the next 5 months of follow-up at the time of publication. At 3 weeks after irradiation, often the peak of treatment reactions, only grade 1 epithelitis and transient grade 1 edema in soft tissues surrounding the tumor was observed. There was no decrease in thickness of the epidermis and no disruption at the basal membrane, with only limited increase in vascularization. Bourhis et al concluded that the first FLASH RT treatment in humans was both effective and safe.30
Rationale for FLASH Delivered with Protons
In its purest form, FLASH RT is merely the use of radiation delivered at a dose rate several orders of magnitude higher than conventional RT. While electron linear accelerators have been used in the aforementioned studies,22,26,30 treatment using electrons has its limitations. Electron FLASH, in its current form and in keeping with conventional-dose rate electron therapy, has low tissue penetration and a general inability to treat deep-seeded tumor volumes, less conformal dose distributions, and limited field size, thereby effectively only allowing the treatment of superficial cancers such as skin cancers and cutaneous lymphomas.31 In contrast, FLASH delivered with proton therapy can overcome this penetration limitation and treat any body depth based on its current delivery approach of transmission FLASH. When delivering transmission FLASH, which is currently the easiest way to deliver FLASH dose rates using proton therapy and which also eliminates uncertainties associated with the positioning of the Bragg Peak that might be magnified with ultrafast delivery of therapy and might result in underdosing of tumor and marginal misses in target, the Bragg peak is intentionally placed outside (behind) the patient such that the proton FLASH target volume is treated with the part of the beam before the Bragg peak.8,32
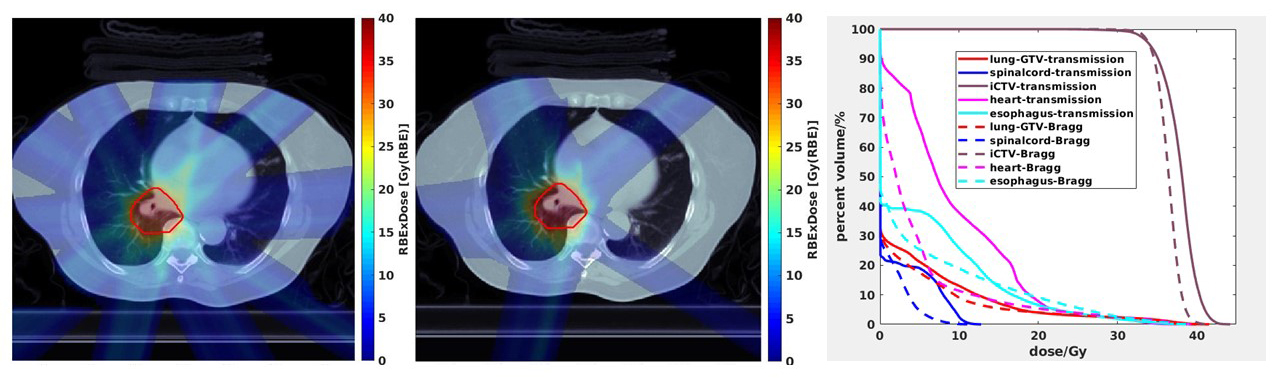
Beyond the currently sizable advantage in depth of penetration, there are additional advantages of using protons to deliver FLASH. Early studies have been conducted investigating how to optimize FLASH delivered with proton therapy,33 including the delivery of Bragg peak plans with the Bragg peak placed in the patient that allows for the elimination of exit dose and a reduction of irradiation beyond the tumor volume as opposed to transmission beams to the tumor (Figure 1). Proton FLASH could have both biological and physical advantages in achieving the FLASH effect with a high linear energy transfer while also administering the majority of its beam energy into a narrow range of the Bragg peak and sparing normal tissues beyond the target volume, respectively.21 The physical advantages of proton FLASH using Bragg peak planning relative to electron FLASH or proton FLASH using transmission planning might be magnified when needing to treat to higher doses for tumor control. Furthermore, proton accelerators are currently much better suited to deliver FLASH RT over photon and electron linear accelerators that would require significant machine manipulation to attain the ultrahigh dose rates needed to achieve the FLASH effect and that also suffer from field size restrictions. In acknowledgment, the first-in-human clinical trial investigates the feasibility of FLASH RT delivered with protons.
First-in-Human Clinical Trial
As of this writing (April 2021), the first-ever FLASH RT human clinical trial underway is the only one initiated to date (ClinicalTrials.gov Identifier: NCT04592887, first posted 10/19/20). The trial, Feasibility Study of FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases (FAST-01), is being conducted at the Cincinnati Children’s Proton Therapy Center. The target sample size for this open label, single-arm prospective feasibility study is 10 patients at least 18 years old with up to 3 painful bone metastases in the extremities who are estimated to have a life expectancy of at least 2 months. As of April 15, 2021, 4 patients have been accrued who received palliative FLASH RT. The primary endpoints are workflow feasibility and radiation-related toxicities; secondary endpoints are pain relief, pain flare, and use of pain medication. No interim results are available at this time.
In this first-in-human trial, patients are being treated in a single fraction with FLASH proton therapy to 8 Gy using a single transmission beam and predefined treatment field size. The target is the gross tumor volume, and a margin of 0.5 cm or more is used. The duration of the fraction is less than a second, and the dose rate is 40 Gy/s or greater.
Proton FLASH Trial Considerations
While several studies provide insights into the mechanism of action for FLASH RT, great uncertainty still exists. Further preclinical research is needed to investigate the mechanism of action, and this work and other preclinical work are needed to help inform and refine future clinical trials.34,35 Moreover, ongoing studies of FLASH RT in veterinary cancer patients can complement and extend the mechanistic insights gained from these preclinical studies.
Aside from the ongoing trial in Cincinnati, additional human studies are needed and are being developed. These trials should be conducted in sites with strong preclinical evidence supporting the FLASH effect with tissue sparing and without tumor protection. Furthermore, as FLASH has the potential to be a transformative treatment modality, its benefit may be most significantly seen in disease sites with high toxicities, particularly those with poor local control. As such, endpoints of future studies should be selected in which a clear difference between FLASH and non-FLASH regimens can be identified should a difference exist, such as the reduction of high-grade toxicities.
Locally advanced non-small cell lung cancer (NSCLC) may be a suitable target – it has one of the highest normal tissue toxicity burdens across all cancers, and its 5-year survival rate is approximately only 30% to 40%.36-39 Furthermore, dose escalation may improve local control and, thereby, overall survival when delivered safely.40 However, this disease site has unique challenges of tumor motion with respiration. Intrafractional motion is a clinical concern with conventional fractionation and can result in an interplay effect with conventional-dose rate RT treatment delivery.41 While less of an issue with transmission planning, this same interplay effect futher challenges the delivery of FLASH when using Bragg peak planning, although these concerns may be less significant since FLASH RT is anticipated to be delivered in a fraction of a second per beam and in a single or just a few fractions. Breath-hold strategies, therefore, may be particularly important when planning such FLASH trials for thoracic and upper gastrointestinal malignancies.
Early stage NSCLC, especially for central or ultracentral tumors that have considerably higher rates of toxicities from stereotactic body radiation therapy (SBRT) than peripheral tumors, may also be a suitable target. Aside from similar concerns of delivering FLASH to a moving target and in a curative population for an early clinical trial, study accrual may be relatively easier due to the large and increasing number of patients diagnosed with early stage NSCLC. FLASH may be an optimal approach for these central tumors given that there is currently no definitive standard-of-care treatment with traditional dose rate RT, especially for ultracentral lesions, and given the high toxicity and even mortality rates with current RT approaches.42-45
Thoracic metastases may also be appealing due to relatively high toxicity rates seen when delivering RT to the chest in patients who are on or have received several lines of systemic therapy, as well as the large volume of patients with intrathoracic metastases and corresponding ease of accrual. Furthermore, surgery or alternative ablative therapies can be salvage options if FLASH RT does not lead to adequate local control or symptomatic response. However, heterogeneous histologies and heterogeneous systemic therapies, including the potential for concurrent chemotherapy or immunotherapy, along with tumor motion, may complicate such trials.
Other notable sites include glioblastoma multiforme, hepatocellular carcinoma, and locally advanced pancreatic cancer, each of which is a common malignancy with high toxicity rates and guarded overall prognoses. Glioblastoma multiforme has failure patterns that are predominantly local, although prior attempts at dose escalation did not improve tumor control or survival.46,47 Hepatocellular carcinoma similarly has failure patterns that are predominantly local before distant, but dose escalation can improve local control.48,49 Furthermore, conventional RT treatments for liver tumors are currently limited by the inherent radiosensitivity of hepatocytes and the risks of radiation-induced liver toxicities. As such, FLASH holds the potential to improve the therapeutic ratio for these challenging tumors. However, no preclinical FLASH RT studies have been performed in liver tumors to date. Patients may be on heterogeneous systemic therapies, and tumor motion is also a factor. Direct visualization of the tumor prior to treatment may be more challenging as well. Likewise, there are no preclinical FLASH RT studies for locally advanced pancreatic cancer, and the potential for duodenal toxicity may prove challenging for early clinical trials in FLASH, in addition to concerns of systemic therapies, tumor motion, and pretreatment visualization. Lastly, consideration should be given to clinical trials in patients receiving preoperative FLASH therapy, such as for sarcoma, to gain insights into the biological effects of FLASH in resected tissue specimens.
Additional Trial Considerations
Several other considerations are critical when considering future human administration of FLASH RT. It is important to ensure that all or most regions of the treatment field receive dose rates above what is considered the threshold for the FLASH effect, as critical normal tissue treated at very high dose rates, but at rates insufficient to achieve the FLASH effect, could actually worsen the therapeutic ratio. Furthermore, it is unclear whether there is a differential effect with higher FLASH RT dose rates; further investigation, both in preclinical and clinical settings, are needed to determine whether 40 Gy/s is adequate, or if dose rates of 80 to 120 Gy/s or more are preferable. Additionally, preclinical data are needed assessing FLASH effects with fractionation, akin to conventional-dose rate SBRT, as current FLASH data have focused on single fraction delivery.
There also needs to be careful deliberation over the total irradiation per voxel, the number of times a voxel gets irradiated, and the overlapping of beams. Processes must likewise be developed and enacted for the scenario in which a treatment interruption might occur in the middle of a beam, as well as methods to ensure dose rate can be reliably measured at the ultrahigh dose rates used for FLASH RT. Additionally, development of Bragg peak delivey of FLASH is indicated to further optimize dose comformality and to allow for the FLASH treatment size to not be limited by the maximum beam energy, thus expanding the areas deliverable with FLASH to deep tumors such as gastrointestinal target volumes that might be challenging to treat with transimission FLASH plans. Of final note, successful delivery of FLASH RT is also contingent on technological advancement, including improved ease of delivering FLASH with current linear accelerators that at present require significant machine manipulation.
Conclusion
FLASH RT is a promising treatment option, but much research utilizing this technology is still in its infancy, and limited animal and human data exist. If it proves to have the normal tissue- sparing effects as demonstrated in multiple early preclinical reports, FLASH is poised to result in a significant evolution in the field of oncology.
References
- Simone CB 2nd. Thoracic radiation normal tissue injury. Semin Radiat Oncol. 2017;27:370-377.
- Kaiser A, Eley JG, Onyeuku NE, et al. Proton therapy delivery and its clinical application in select solid tumor malignancies. J Vis Exp. 2019;144.
- Chun SG, Solberg TD, Grosshans DR, et al. The potential of heavy-ion therapy to improve outcomes for locally advanced non-small cell lung cancer. Front Oncol. 2017;7:201.
- Patel SH, Wang Z, Wong WW, et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014;15:1027-1038.
- Lin SH, Hobbs BP, Verma V, et al. Randomized phase IIB trial of proton beam therapy versus intensity-modulated radiation therapy for locally advanced esophageal cancer. J Clin Oncol. 2020;38:1569-1579.
- Higgins KA, O’Connell K, Liu Y, et al. National Cancer Database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97:128-137.
- Sanford NN, Pursley J, Noe B, et al. Protons versus photons for unresectable hepatocellular carcinoma: liver decompensation and overall survival. Int J Radiat Oncol Biol Phys. 2019;105:64-72.
- Kahalley LS, Peterson R, Ris MD, et al. Superior intellectual outcomes after proton radiotherapy compared with photon radiotherapy for pediatric medulloblastoma. J Clin Oncol. 2020;38:454-461.
- Baumann BC, Mitra N, Harton JG, et al. Comparative effectiveness of proton vs photon therapy as part of concurrent chemoradiotherapy for locally advanced cancer. JAMA Oncol. 2020;6:237-246.
- Verma V, Simone CB 2nd, Mishra MV. Quality of life and patient-reported outcomes following proton radiation therapy: a systematic review. J Natl Cancer Inst. 2018;110.
- Doyen J, Falk AT, Floquet V, Hérault J, Hannoun-Lévi JM. Proton beams in cancer treatments: clinical outcomes and dosimetric comparisons with photon therapy. Cancer >Treat Rev. 2016;43:104-112.
- Hoppe BS, Nichols RC, Flampouri S, et al. Hypofractionated proton therapy with concurrent chemotherapy for locally advanced non-small cell lung cancer: a phase 1 trial from the University of Florida and Proton Collaborative Group. Int J Radiat Oncol Biol Phys. 2020;107:455-461.
- Verma V, Rwigema JM, Malyapa RS, Regine WF, Simone CB 2nd. Systematic assessment of clinical outcomes and toxicities of proton radiotherapy for reirradiation. Radio>ther Oncol. 2017;125:21-30.
- Wilson JD, Hammond EM, Higgins GS, Petersson K. Ultra-high dose rate (FLASH) radiotherapy: silver bullet or fool’s gold? Front Oncol. 2020.
- Smyth LML, Donoghue JF, Ventura JA, et al. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci Rep. 2018;8:12044.
- Wilson P, Jones B, Yokoi T, Hill M, Vojnovic B. Revisiting the ultra-high dose rate effect: implications for charged particle radiotherapy using protons and light ions. Br J Radiol. 2012;85:e933-e939.
- Kim YE, Gwak SH, Hong BJ, et al. Effects of ultra-high doserate FLASH irradiation on the tumor microenvironment in lewis lung carcinoma: role of myosin light chain. Int J Radiat Oncol Biol Phys. 2021;109(5):1440-1453.
- Narayanan PK, Rudnick JM, Walthers EA, Crissman HA. Modulation in cell cycle and cyclin B1 expression in irradiated HeLa cells and normal human skin fibroblasts treated with staurosporine and caffeine. Exp Cell Res. 1997;233:118-127.
- Auer S, Hable V, Greubel C, et al. Survival of tumor cells after proton irradiation with ultra-high dose rates. Radiat Oncol. 2011;6:139.
- Buonanno M, Grilj V, Brenner DJ. Biological effects in normal cells exposed to FLASH dose rate protons. Radiother Oncol. 2019;139:51-55.
- Hughes JR, Parsons JL. FLASH radiotherapy: current knowledge and future insights using proton-beam therapy. Int J Mol Sci. 2020;21:6492.
- Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Trans Med. 2014;6:245ra93.
- Diffenderfer ES, Verginadis II, Kim MM, et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int J Radiat Oncol Biol Phys. 2020;106:440-448.
- Chabi S, To THV, Leavitt R, et al. Ultra-high-dose-rate FLASH and conventional-dose-rate irradiation differentially affect human acute lymphoblastic leukemia and normal hematopoiesis. Int J Radiat Oncol Biol Phys. 2020;109:819-29.
- Spencer JA, Ferraro F, Roussakis E, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269-273.
- Vozenin MC, De Fornel P, Petersson K, et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res. 2019;25:35-42.
- Bourhis J, Montay-Gruel P, Gonçalves Jorge P, et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother Oncol. 2019;139:11-17.
- Montay-Gruel P, Petersson K, Jaccard M, et al. Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol. 2017;124:P365-369.
- Lohse I, Lang S, Hrbacek J, et al. Effect of high dose per pulse flattening filter-free beams on cancer cell survival. Radiother Oncol. 2011;101:226-232.
- Bourhis J, Sozzi WJ, Jorge PG, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 2019;139:P18-22.
- Montay-Gruel P, Meziani L, Yakkala C, Vozenin MC. Expanding the therapeutic index of radiation therapy by normal tissue protection. Br J Radiol. 2019;92:20180008.
- Zou W, Diffenderfer ES, Cengel KA, et al. Current delivery limitations of proton PBS for FLASH. Radiother Oncol. 2021;155:212-218.
- Verhaegen F, Wanders RG, Wolfs C, Eekers D. Considerations for shoot-through FLASH proton therapy. Phys Med Biol. 2021;66:06nt1.
- Breneman J, Perentesis JP, Bradley J, et al. Methodical approach to FLASH clinical trials: a comment on Buchsbaum et al., FLASH radiotherapy: new technology plus biology required. Int J Radiat Oncol Biol Phys. 2021.
- Buchsbaum JC, Coleman CN, Espey MG, et al. FLASH radiotherapy: new technology plus biology required. Int J Radiat Oncol Biol Phys. 2021.
- Verma V, Simone CB 2nd, Werner-Wasik M. Acute and late toxicities of concurrent chemoradiotherapy for locally-advanced non-small cell lung cancer. Cancers (Basel). 2017;9:120.
- Kong F-MS, Zhao J, Wang J, Faivre-Finn C. Radiation dose effect in locally advanced non-small cell lung cancer. J Thorac Dis. 2014;5:336-347.
- Vrankar M, Stanic K. Long-term survival of locally advanced stage III non-small cell lung cancer patients treated with chemoradiotherapy and perspectives for the treatment with immunotherapy. Radiol Oncol. 2018;52:281-288.
- Faivre-Finn C, Vicente D, Kurata T, et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC-an Update from the PACIFIC trial. J Thorac Oncol. 2021;16:860-867.
- Ma L, Men Y, Feng L, et al. A current review of dose-escalated radiotherapy in locally advanced non-small cell lung cancer. Radiol Oncol. 2019;53:6-14.
- Kang M, Huang S, Solberg TD, et al. A study of the beam-specific interplay effect in proton pencil beam scanning delivery in lung cancer. Acta Oncol. 2017;56:531-540.
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2017;28:IV1-21.
- Abel S, Hasan S, Horne ZD, Colonias A, Wegner RE. Stereotactic body radiation therapy in early-stage NSCLC: historical review, contemporary evidence and future implications. Lung Cancer Manag. 2019;8:LMT09.
- Simone CB 2nd, Wildt B, Haas AR, Pope G, Rengan R, Hahn SM. Stereotactic body radiation therapy for lung cancer. Chest. 2013;143:1784-1790.
- Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with “ultracentral” non-small cell lung cancer. J Thorac Oncol. 2016;11:1081-1089.
- Badiyan SN, Markovina S, Simpson JR, et al. Radiation therapy dose escalation for glioblastoma multiforme in the era of temozolomide. Int J Radiat Oncol Biol Phys. 2014;90:877-885.
- Wegner RE, Abel S, Horne ZD, et al. National trends in radiation dose escalation for glioblastoma. Radiat Oncol J. 2019;37:13-21.
- Byun HK, Kim HJ, Im YR, Kim DY, Han KH, Seong J. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma. Radiother Oncol. 2019;133:1-8.
- Herrmann E, Naehrig D, Sassowsky M, et al. External beam radiotherapy for unresectable hepatocellular carcinoma, an international multicenter phase I trial, SAKK 77/07 and SASL 26. Radiat Oncol. 2017;12:12.
Citation
R C, M K, S W, JI C, RH P, S H, AM C, KA C, H L, Simone, II C. FLASH Radiation Therapy: Review of the Literature and Considerations for Future Research and Proton Therapy FLASH Trials. Appl Radiat Oncol. 2021;(2):15-21.
July 27, 2021