GammaTile A “One-And-Done” Radiation Option for Patients with Brain Tumors
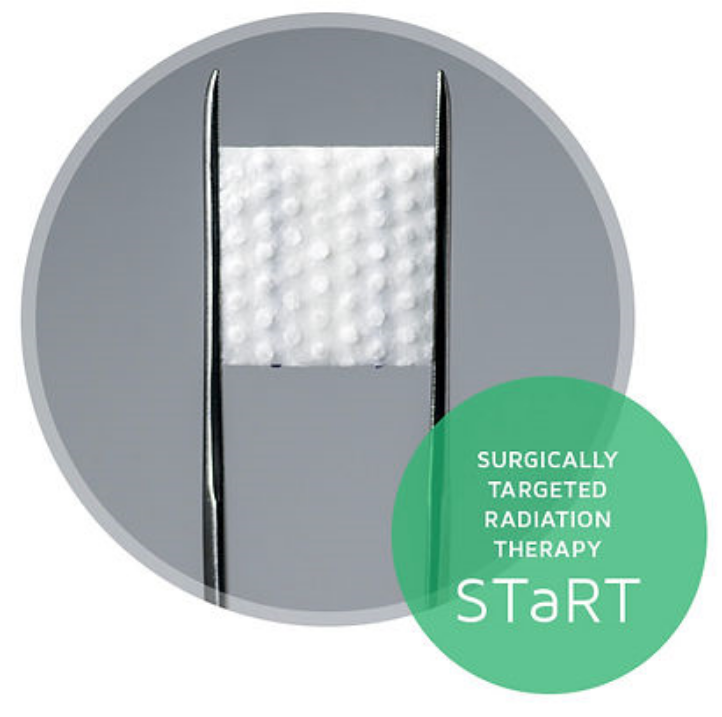 New data from GT Medical Technologies details how Surgically Targeted Radiation Therapy (STaRT) provides “one-and-done” radiation treatment at the time of brain tumor removal.
New data from GT Medical Technologies details how Surgically Targeted Radiation Therapy (STaRT) provides “one-and-done” radiation treatment at the time of brain tumor removal.
GT Medical Technologies is presenting this clinical data in two oral presentations on the company's breakthrough GammaTile Therapy for patients with brain tumors at the 2021 American Association of Neurological Surgeons (AANS) Annual Scientific Meeting. The presenting authors, Dr. David Brachman, co-founder and CTO at GT Medical Technologies and Dr. Mehee Choi, director of Medical Affairs at GT Medical Technologies, will discuss new data in patients with recurrent glioblastoma (GBM), as well as access to care for patients with resectable brain tumors.
GammaTile is an implantable radiation therapy consisting of bioresorbable collagen tiles embedded with Cesium-131 sources provided by Isoray. It’s the first medical device cleared for brain tumor treatment in the US in the last 10 years.
“GammaTile Therapy improves the lives of patients with newly diagnosed malignant and recurrent brain tumors,” said Matthew Likens, president and CEO of GT Medical Technologies. “Data presented at AANS highlights clinical efficacy of the therapy, but also important access-to-care and quality-of-life benefits that GammaTile provides these patients. It's the first device of its kind, and its ability to deliver safe and effective Surgically Targeted Radiation Therapy (STaRT) for patients is groundbreaking in the neuro-oncology community.”
The in-person AANS meeting was cancelled due to COVID-19. As the pandemic disrupts travel and events, it’s vital to remember that cancer treatment is not elective. GammaTile is a one-and-done radiation option for patients with brain tumors that eliminates the need for daily, ongoing radiation treatments. Patients receive ongoing, therapeutic radiation from the safety of their own home, protecting them from unnecessary exposure to COVID-19, and delivering life-extending treatment.