High dose rate brachytherapy for prostate cancer: Current techniques and applications to varying disease presentations
Images
SAM-CME credits available here
High dose rate (HDR) brachytherapy has been an option for managing localized prostate cancer since the early 1990s. Several features of this treatment approach make it attractive to both patients and clinicians. First, the convenience of brachytherapy in general—namely, the potential to significantly shorten or eliminate the need for daily external-beam radiation therapy (EBRT) treatment visits that may extend up to 2 months—holds appeal to many younger, healthy, active patients looking to definitively address their disease with minimal disruption to their daily routine. Prostate brachytherapy options include permanent prostate seed implant, or low dose rate (LDR), brachytherapy and temporary prostate implant, more commonly called HDR brachytherapy. Both types of brachytherapy are safe and effective across a range of clinical presentations of prostate cancer and have been applied relatively consistently. Each may be used as definitive therapy, as is commonly the case for men with more indolent prostate cancers, or in conjunction with an abbreviated course of pelvic EBRT for men with more aggressive disease presentations.
Relative to LDR, or permanent seed, brachytherapy, HDR offers several advantages including: no patient-specific radiation precautions having to be implemented as patients are not radioactive following treatment completion; decreased radiation exposure to clinical staff and the general public; increased clinical control over dose administration; and exploitation of the perceived sensitivity of prostate cancer to large, individual fractional doses1,2 of radiation in contrast to the gradual deposition of dose over many months, as is the case with permanent seed implants. Each of these factors holds potential appeal to clinicians and patients and has contributed to heightened implementation of HDR brachytherapy over the past 20-plus years.
No direct clinical evidence exists supporting HDR brachytherapy’s superiority over LDR (or vice versa) in terms of improved tumor control or reduced toxicity as there has never been a prospective, randomized comparison between the approaches. There are, however, several disadvantages to prostate HDR brachytherapy that may make it less appealing to practitioners depending on a given practice infrastructure, personnel availability, and time constraints. First, LDR procedures use low-energy radiation sources, most commonly iodine 125 (125I) or palladium 103 (103Pd). As such, these procedures require minimal source shielding, can be handled directly by clinical staff, and may be placed in a standard operating room. Iridium 192 (192Ir), the most common HDR brachytherapy source, is high energy and requires a shielded vault for treatment. Logistically, this necessitates that interstitial implant procedures be performed in a shielded operating room and, if not available, that the interstitial implant will be left in the patient while he is moved for treatment planning and administration procedures. This increases time demands on physicians and ancillary clinical staff, and poses additional workflow challenges regarding anesthesia and quality assurance steps to minimize risks of implant displacement as patients are moved. Lastly is the Nuclear Regulatory Commission requirement that the treating radiation oncologist be physically present for treatment administration, which further increases the physician’s time commitment.
Despite logistical challenges, the clinical advantages have held sufficient appeal to physicians and patients, such that it is becoming increasingly offered to patients as a viable treatment option for prostate cancer. Its safety and efficacy have been documented in multiple large prospective and retrospective institutional series, and ongoing investigation continues to streamline treatment approaches, bolstering convenience for radiation oncology departments and improving patient accessibility.
Patient Selection and Technical Description
Successful implementation of HDR brachytherapy for prostate cancer begins with appropriate patient selection. Anatomic factors lending to successful HDR treatment are as follows: prostate volume of approximately 20-60 cc (glands outside this range may still be considered for treatment); a central/straight urethral position that can be adequately avoided during transperineal needle implant; absence of significant benign prostatic hypertrophy/median lobe or transurethral resection of the prostate (TURP) defect at the prostate base; adequate spacing between the prostate and rectum; and adequate pubic arch width to avoid interference with needle placement. Clinical factors include general risks of both anesthesia and elective surgical procedures (the latter of which include comorbidities such as diabetes, cardiopulmonary factors, coagulopathy, etc.). Careful attention must be paid to baseline urinary function. Brachytherapy is not a good treatment option for patients with significant baseline obstructive uropathy. Risks for significant obstructive complications of brachytherapy increase substantially in such patients, and our practice typically will exclude patients with a baseline American Urologic Association (AUA) symptom score > 15 if already on medication, or > 20 if previously untreated for obstructive symptoms.3 Any of these factors are relative contraindications to performing HDR brachytherapy and the treating physician should consider them on a patient-by-patient basis, weighing risks and benefits.
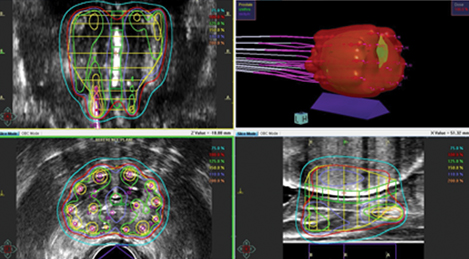
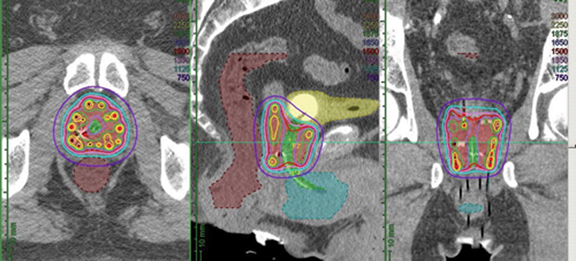
HDR brachytherapy procedures all begin with a transrectal ultrasound-guided transperineal implant of the prostate gland. A typical implant will consist of 15-20 needles placed symmetrically throughout the prostate, after which image-based dosimetric planning will be performed. Dosimetry may be calculated by images acquired directly on the transrectal ultrasonography (TRUS) unit used for needle implantation, computed tomography (CT) scan, or even MRI. Ideally, coverage of the prostate gland with the prescription isodose should exceed 95% of its volume (V100 > 95%). Typical dose heterogeneity tolerances are as follows: volume of prostate receiving 125% and 150% of the prescription dose (V125 and V150, respectively) should be < 60% and < 30%, respectively. Ideally, efforts should be made to avoid “hot spots” in the urethra, typically keeping < 5% of the urethra under 110% of the prescribed dose. Maximum dose to 1 cc of the rectum dose should not exceed 75% of the dose prescribed. Although typically not quantified for ultrasound-planned cases, a similar bladder constraint of < 1 cc receiving 75% of the prescription dose is typically applied for CT-based dosimetry. Figure 1 shows representations of an ultrasound- and CT-planned case.
Advantages and disadvantages are associated with each approach, and none have been shown to be clinically superior. Advantages to ultrasound-based planning include precise visualization of the prostate capsule and, should the infrastructure exist for a shielded operating room, the potential to complete an entire brachytherapy treatment administration without having to move the patient from the time of needle placement to dose delivery. CT-based dosimetry affords the opportunity for more precise anatomic quantification of bladder and rectal doses but requires that patients be moved multiple times to complete imaging studies and then returned to the shielded treatment room for treatment delivery. MRI is being used for dose planning in selected centers and, while providing unequivocally the highest image quality for dose planning, presents additional challenges regarding MR compatibility of the prostate implant and any necessary anesthesia equipment needed while the implant is in place.4.5
Once treatment planning is complete, the interstitial needles are connected to a remote afterloader that will deliver the radiation dose through each needle via an192Ir source. Depending on prostate size, dose prescribed, activity of the source, etc., treatment delivery is usually completed in 15-25 minutes. The implant may then be removed from the patient if the treatment given is the final prescribed fraction or secured for delivery of subsequent treatment fractions. Interfraction treatment interval should generally exceed a minimum of 6 hours.
HDR in Conjunction with External Beam
When first used for prostate cancer, HDR brachytherapy was predominantly implemented to boost the prostate as an adjunct to pelvic EBRT. This was considered particularly advantageous when first being used given the contemporary EBRT doses of 66-70 Gy that were considered standard at the time. Several single-institution experiences reported on the safety and efficacy of such treatment, and a prospective, randomized trial published by Hoskin et al6 demonstrated superior biochemical control in patients receiving HDR brachytherapy boost treatment relative to those patients treated with EBRT alone.
Biological analyses of responses to changes in HDR dosing/fractionation suggested a high sensitivity of prostate cancer cells to increasing fractional doses. That is, prostate cancer has a low alpha-beta ratio, and biologically equivalent dose (BED), it was found, could be dramatically increased through relatively small increases in HDR fraction size. In fact, evidence of dose response in terms of enhanced biochemical control was demonstrated across a range of HDR fractionation regimens increasing progressively from as low as 550 cGy x 3 fractions up to 1150 cGy x 2 fractions.7 More recently, HDR boost regimens given in a single fraction of 1500 cGy (usually in the context of ~45 Gy given to the pelvis via EBRT)8,9 has gained favor and is the recommended dosing in the most recent open Radiation Therapy Oncology Group protocol (RTOG 0924) to allow HDR brachytherapy as a boost.
Despite the randomized evidence from Hoskin et al, a lack of evidence remains that conclusively supports the benefits of the combined approach of EBRT with HDR brachytherapy over contemporary, dose-escalated EBRT alone. Namely, a significant criticism of the Hoskin randomized trial has been, and remains, that the EBRT dose (55 Gy in 20 fractions) would be considered substandard in light of multiple randomized trials demonstrating biochemical control advantages for EBRT doses of 78-80 Gy.10-12 Nonetheless, outcomes from that study revealed significantly improved median time to relapse of 116 months vs. 74 months favoring the group receiving HDR brachytherapy; this was despite a relatively modest HDR boost dose level of 8.5 Gy x 2.
Just recently, however, the ASCENDE-RT (Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy) prospective randomized trial has delivered corroborating evidence that adding LDR brachytherapy to pelvic EBRT improves biochemical control compared to men receiving 78 Gy EBRT alone for men with intermediate- or high-risk prostate cancer.13 In that study, patients receiving EBRT alone were twice as likely to experience biochemical failure as those receiving an LDR brachytherapy boost (9-year biochemical control 83% vs. 62%; p < 0.001). The improvement in biochemical control did, however, come at a cost of increased toxicity. Five-year rates of grade 3 genitourinary (GU) toxicity were 18.4% for the brachytherapy patients compared to 5.2% for the dose-escalated EBRT patients, and 5-year grade 3 GI toxicity rates were 8.1% vs. 3.2%, respectively, for the brachytherapy boost vs. EBRT alone patients.14
No prospective, randomized comparisons support a biochemical or clinical disease control advantage for patients receiving HDR brachytherapy over contemporary, dose-escalated EBRT. However, single-institution experiences have reported favorable disease control rates comparable to those in the ASCENDE-RT trial with more favorable toxicity profiles. In a series of 832 intermediate- and high-risk disease patients treated with variable doses of HDR brachytherapy boost in conjunction with EBRT, Vigneault et al reported chronic grade 3 GU toxicity rates of 1.9-4.7% based on the dose levels to which patients were treated, and there was no reported acute or chronic grade 3 GI toxicity.15 Biochemical control was approximately 95%. Martinez et al reported on the benefits of dose escalation using HDR brachytherapy as a boost. For the 472 patients described, chronic grade 3 GU toxicity rates were approximately 1% while reporting favorable 10-year biochemical control of approximately 81% for patients treated to high dose levels.7 Yaxley et al reported 10-year biochemical control rates of 87% and 56% for intermediate- and high-risk patients, respectively, while showing that urethral stricture rate can be markedly reduced through careful attention to dose heterogeneity constraints, control for needle displacement prior to HDR treatment, and tighter inferior PTV margins during the EBRT portion of therapy.16
Finally, although a detailed discussion of androgen suppression is beyond the scope of this review, it should be mentioned that despite the aggressive disease presentations addressed with HDR boost therapy, the benefits of androgen suppression in the context of such treatment appears to be minimized or even absent.17,18 To be clear, the role for androgen suppression has never been tested in prospective randomized fashion for HDR boost patients as it has for patients receiving EBRT treatment. Although this issue warrants additional consideration going forward, current standard practice, in this author’s opinion, includes the administration of androgen suppression with HDR boost for patients with high-risk disease features.
HDR Brachytherapy as Monotherapy
For patients with more favorable presenting disease characteristics, HDR brachytherapy alone (no EBRT) may be used as definitive local therapy for men with low- or favorable intermediate-risk disease. This treatment approach holds great appeal as treatment may be completed typically in 1 or, at most, 2 minimally invasive, outpatient procedures with no daily attendance requirement for external beam administration. Recent updates of large, single-institution experiences have revealed highly favorable disease control rates across a range of treatment techniques and fractionation regimens. Additionally, toxicity rates have proven highly favorable and, most notably, with optimal techniques and the elimination of supplementary EBRT, rectal toxicity rates grade > 2 are remarkably low. Using HDR as monotherapy for favorable and intermediate-risk prostate cancer requires a greater level of technical/planning expertise to ensure adequate target coverage relative to its use as a boost adjunct to pelvic external beam treatment. As such, HDR used as monotherapy has been considered investigational in published guidelines by both the American Brachytherapy Society19 and the Groupe Européen de Curiethérapie (GEC) and the European Society for Radiotherapy & Oncology (ESTRO).20 The most recent publication of these guidelines, however, are 4-5 years old, and since that time, multiple large, single-institution experiences have described highly favorable outcomes for this approach. As such, it has gained favor in the hands of experienced practitioners as a standard option for patients with favorable or intermediate-risk prostate cancer.
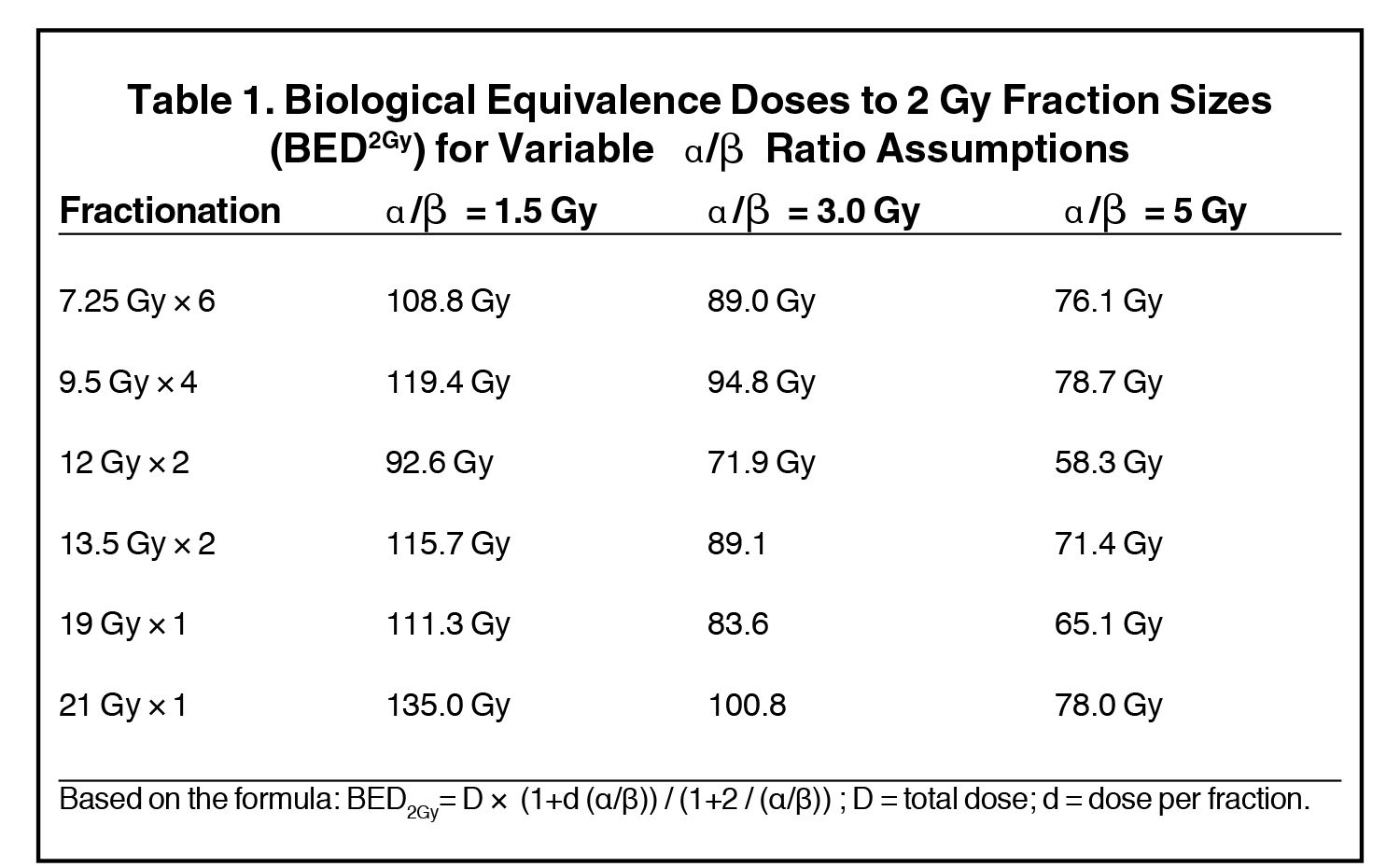
A variety of dosing/fractionation regimens have been explored and reported on previously: 950 cGy × 4, 1200 cGy × 2, 1350 cGy × 2, 700 cGy × 6, 725 cGy × 6, 6 Gy × 8, 6 Gy × 9, and 6.5 Gy × 7 with similarly high biological equivalence to standard 2-Gy treatment fractions as shown in Table 1. William Beaumont Hospital compared treatment toxicity and outcomes of 494 low- and intermediate-risk patients treated with 1 of 3 dosing regimens: 950 cGy x 4, 1200 cGy × 2, and 1350 cGy × 2. Five-year biochemical control rates were 97%, 87%, and 93%, respectively, with no statistically significant differences appreciated between the treatment arms.21 Of note, a significantly higher percentage of patients treated with the 950 cGy × 4 fractions regimen was considered NCCN (National Comprehensive Cancer Network) low-risk relative to the 2-fraction dosing regimens examined. Chronic grade 3 urinary toxicity rates were < 1% for all patients, and no chronic grade ≥ 3 GI toxicities were reported. UCLA recently reported on a similar cohort of 460 low- (64%) and intermediate-risk (36%) prostate cancer patients treated with HDR brachytherapy as monotherapy treated with doses of 42-43.5 Gy delivered in 6 treatment fractions over 2 implant procedures. Kaplan-Meier estimates of biochemical control rates were excellent at 98.9% for low-risk and 95.2% for intermediate-risk patients at 10 years.22 No grade ≥ 3 rectal toxicity was reported, and chronic grade ≥ 3 urinary toxicity was < 1%. Additionally, Yoshioka et al reported out of Japan favorable outcomes in a series of 190 patients with intermediate- (n = 79) and high-risk (n = 111) prostate cancer treated with combination androgen suppression and HDR brachytherapy without the addition of EBRT. Historically, such patients would be offered HDR in combination with EBRT, yet reported outcomes were highly favorable. Using variable multifraction dosing regimens (6 Gy x 8; 6 Gy x 9; or 6.5 Gy x 7) given over 4-5 days, biochemical control rates reported at 8 years were 91% and 77%, respectively, for the intermediate- and high-risk patient subsets.23 Similar to the previously described series, late severe toxicity was rare. Four grade 3 toxicity events in the series were reported: 2 urinary (1 incidence of hematuria and 1 urinary tract obstruction) and 2 GI (sigmoid colon perforation and urethrorectal fistula).
As clinicians have grown more comfortable with administering HDR brachytherapy in definitive fashion for prostate cancer, the management trend has been to increase fractional doses and decrease overall treatment fractions. This has been evident as common practice has trended from 4-6 fraction regimens down to 2. More recently, this has been taken to the extreme with several series reporting outcomes of patients treated entirely in a single HDR brachytherapy treatment fraction. Hoskin et al was the first to report outcomes of patients treated with doses of 19 Gy or 20 Gy in a single fraction.24 Despite relatively favorable toxicity rates in this series, an increased rate of catheter usage was noted in the patients receiving 20 Gy as compared to those receiving 19 Gy. In a subsequent publication, patients with more aggressive prostate cancers (74% to 87% receiving supplemental androgen suppression) receiving single-fraction HDR brachytherapy as monotherapy were found to have similar long-term toxicity and biochemical control rates compared to patients treated using a 2- or 3-fraction regimen (13 Gy × 2; 10.5 Gy × 3).25 A series of 60 patients treated with a single 19 Gy HDR fraction reported by Prada et al yielded highly favorable toxicity rates with no acute or chronic ≥ grade 2 urinary toxicity reported.26 No significant rectal toxicity was encountered either, but unfavorable 6-year biochemical control rates of 66% and 60% for low- and intermediate-risk patients, respectively, must be considered. William Beaumont Hospital reported on a series of 58 patients treated with 19 Gy in a single fraction. Again, toxicity rates were highly favorable with chronic grade 2 GU toxicity of 12% and no grade 3 GU toxicity recorded. Aside from an isolated incidence of late grade 3 diarrhea requiring hospitalization, no grade ≥ 2 GI toxicity was observed. Preliminary biochemical control (3 years) was reported at 93%.27 The highly favorable toxicity profile and tolerability of single-fraction HDR monotherapy have been corroborated in a prospective randomized comparison demonstrating no significant increase in complication risks relative to a multifraction regimen of 13.5 Gy x 2. In fact, urinary toxicity was slightly increased in the multifraction arm during year 1, and single-fraction treatment was associated with a lower occurrence of ≥ grade 2 erectile dysfunction.28
Despite promising initial results in the single-fraction experience, such treatment remains investigational, and the optimal single-fraction dosing regimen continues to be investigated. Specifically, the 19 Gy single-fraction dose was predicated on the assumption of the extremely low alpha-beta ratio for prostate cancer (1.2-1.5 Gy). Evidence shows that radioresponsiveness of prostate cancer may be heterogeneous and that certain cancers may, in fact, have alpha-beta ratios that are higher.28 For such tumors, 19 Gy may prove to be an insufficient dose (see Table 1), and continued evaluation of the optimal single-fraction HDR treatment approach is necessary.
Summary
HDR brachytherapy for prostate cancer is an excellent treatment option for selected patients seeking definitive radiotherapeutic management. Use of HDR as a boost for patients with aggressive prostate cancer has been associated with high disease control rates and favorable toxicity profiles. Based on evidence, favored dosing regimens would be either: HDR brachytherapy 21 Gy in 2 fractions (10.5 Gy per fraction) or single fraction 15 Gy, typically combined with 45-50 Gy in 1.8-2.0 Gy fractions using external beam to the prostate and seminal vesicles +/- pelvic lymph nodes. Although not specifically tested against dose-escalated EBRT alone, potential advantages of brachytherapy added to EBRT in general are strongly suggested by the results of the ASCENDE-RT trial. Use of HDR as monotherapy for patients with low- or intermediate-risk prostate cancer is supported by numerous large, single-institution series reporting favorable long-term biochemical control rates with highly favorable toxicity profiles and should be considered a standard treatment option for such patients. As described, many dosing regimens would be acceptable, with the current standard approach at William Beaumont Hospital being 27 Gy delivered in 2 fractions (13.5 Gy per fraction), generally delivered 2 weeks apart. Caution should be exercised in omitting EBRT from the management of patients with high-risk disease features, as HDR monotherapy data for this patient cohort is limited. Single-fraction HDR brachytherapy as monotherapy should be considered investigational at this point and not offered outside the context of a clinical trial as long-term outcome data are lacking and little empiric evidence regarding optimal single-fraction doses exists.
References
- Brenner D, Martinez A, Edmundson G et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6-13.
- Mirabell R, Roberts S, Zubizarreta E, et al. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82:e17-e24.
- Yamada Y, Rogers L, Demanes DJ, et al. American Brachytherapy Society guidelines for high-dose-rate prostate brachytherapy. Brachytherapy. 2012;11:20-32.
- Frank SJ, Mourtada F, Crook J, et al. Use of MRI in low-dose-rate and high-dose-rate prostate brachytherapy from diagnosis to treatment assessment: defining the knowledge gaps, technical challenges, and barriers to implementation. Brachytherapy. 2017; e-pub ahead of print.
- Murgic J, Chung P, Berlin A, et al. Lessons learned using an MRI-only workflow during high-dose-rate brachytherapy for prostate cancer. Brachytherapy. 2016;15:147-155.
- Hoskin P, Rojas A, Bownes P, et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localized prostate cancer. Radiother Oncol. 2012;103:217-222.
- Martinez A, Gonzalez J, Ye H, et al. Dose escalation improves cancer-related events at 10 years for intermediate- and high-risk prostate cancer patients treated with hypofractionated high-dose-rate boost and external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:363-370.
- Shahid N, Loblaw A, Chung HT, et al. Long-term toxicity and health-related quality of life after single-fraction high dose rate brachytherapy boost and hypofractionated external beam radiotherapy for intermediate-risk prostate cancer. Clin Oncol. 2017;29:412-20.
- Lauche O, Delouya G, Taussky D, et al. Single-fraction high-dose-rate brachytherapy using real-time transrectal ultrasound based planning in combination with external beam radiotherapy for prostate cancer: dosimetrics and early clinical results. J Contemp Brachytherapy. 2016;8:104-109.
- Kuban D, Tucker S, Dong L, et al. Long-term results of the MD Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67-74.
- Heemsbergen W, Al-Mamgani A, Slot A, et al. Long-term results of the Dutch randomized prostate cancer trial: impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol. 2014;110:104-109.
- Zeitman A, DeSilvio M, Slater J, et al. Comparison of conventional-dose vs. high-dose conformal radiation therapy in localized adenocarcinoma of the prostate. JAMA. 2005;294:1233-1239.
- Morris WJ, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:275-285.
- Rodda S, Tyldesley S, Morris WJ, et al. ASCENDE-RT: an analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:286-295.
- Vigneault E, Mbodji K, Magnan S, et al. High-dose-rate brachytherapy boost for prostate cancer treatment: different combinations of hypofractionated regimens and clinical outcomes. Radiother Oncol. 2017;124:49-55.
- Yaxley JW, Lah K, Yaxley JP, et al. Long-term outcomes of high-dose-rate brachytherapy for intermediate- and high-risk prostate cancer with a median follow-up of 10 years. BJU Int. 2017;120:56-60.
- Martinez A, Demanes DJ, Galalae R, et al. Lack of benefit from a short course of androgen deprivation for unfavorable prostate cancer patients treated with an accelerated hypofractionated regime. Int J Radiat Oncol Biol Phys. 2005;62:1322-1331.
- Krauss D, Kestin L, Ye H, et al. Lack of benefit for the addition of androgen deprivation therapy to dose-escalated radiotherapy in the treatment of intermediate- and high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:1064-1071.
- Yamada Y, Rogers L, Demanes DJ, et al. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy. 2012;11:20-32.
- Hoskin P, Colombo A, Henry A, et al. GEC/ESTRO recommendations on high dose rate afterloading brachytherapy for localized prostate cancer: an update. Radiother Oncol. 2013;107:325-332.
- Jawad M, Dilworth J, Gustafson G, et al. Outcomes associated with 3 treatment schedules of high-dose-rate brachytherapy monotherapy for favorable-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2016;94:657-666.
- Hauswald H, Kamrava MR, Fallon JM, et al. High-dose-rate monotherapy for localized prostate cancer:10-year results. Int J Radiat Oncol Biol Phys. 2016;94:667-674.
- Yoshioka Y, Suzuki O, Isohashi F, et al. High-dose-rate brachytherapy as monotherapy for intermediate- and high-risk prostate cancer: clinical results for a median 8-year follow-up. Int J Radiat Oncol Biol Phys. 2016;94:675-682.
- Hoskin P, Rojas A, Ostler P, et al. High-dose-rate brachytherapy alone given as two or one fraction to patients for locally advanced prostate cancer: acute toxicity. Radiother Oncol. 2014;110:268-271.
- Hoskin P, Rojas A, Ostler P, et al. Single-dose high-dose-rate brachytherapy compared to two and three fractions for locally advanced prostate cancer. Radiother Oncol. 2017;124:56-60.
- Prada P, Cardenal J, Blanco AG, et al. High-dose-rate brachytherapy as monotherapy in one fraction for the treatment of favorable stage prostate cancer: toxicity and long-term biochemical results. Radiother Oncol. 2016;119:411-416.
- Krauss D, Ye H, Martinez A, et al. Favorable preliminary outcomes for men with low- and intermediate-risk prostate cancer treated with 19-Gy single-fraction high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2017;97:98-106.
- Morton G, Chung H, McGuffin M, et al. Prostate high dose-rate brachytherapy as monotherapy for low and intermediate risk prostate cancer: early toxicity and quality of life results from a randomized phase II clinical trial of one fraction of 19 Gy or two fractions of 13.5 Gy. Radiother Oncol. 2017;122:87-92.
- Vogelius I, Bentzen S. Meta-analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: bad news, good news, or no news? Int J Radiat Oncol Biol Phys. 2013;85:89-94.
Citation
DJ K. High dose rate brachytherapy for prostate cancer: Current techniques and applications to varying disease presentations. Appl Radiat Oncol. 2017;(3):12-17.
September 21, 2017