High-Dose-Rate Brachytherapy (HDRBT) Followed by Concurrent Chemoradiotherapy for Esophageal Adenocarcinoma
Images

Abstract
This is a case report of a patient with unresectable locally advanced esophageal adenocarcinoma treated with high-dose-rate brachytherapy (HDRBT) followed by chemoradiotherapy (CRT) with intensity-modulated radiation therapy (IMRT). The treatment regimen consisted of 3 fractions of weekly 7 Gy HDRBT followed by CRT to 50.4 Gy in 28 fractions. The patient achieved complete clinical response shown by radiological imaging and endoscopic examination after 3 months of treatment completion, and the response was sustained at 18 months based on radiological imaging. Her dysphagia markedly improved from tolerating fluids only at presentation to managing a semisolid diet after the second fraction of brachytherapy and a solid diet while on CRT. While the patient refused further investigation and did not come for follow-up after the 18-month assessment, she reported no symptoms of disease recurrence up to 40 months in our regular verbal communication/follow-up via telephone.
Keywords: Esophageal brachytherapy, dysphagia, esophageal adenocarcinoma
Case Summary
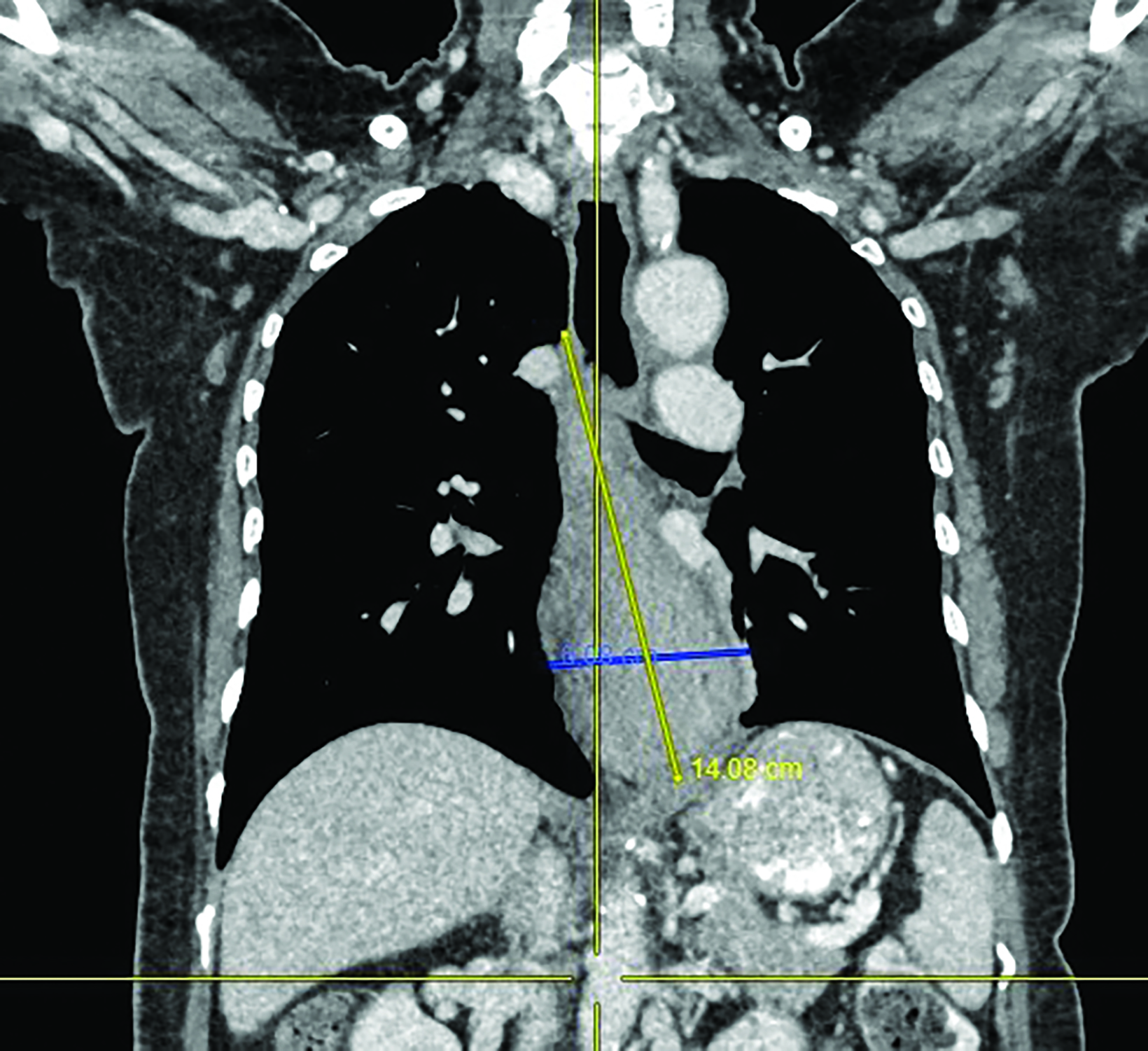
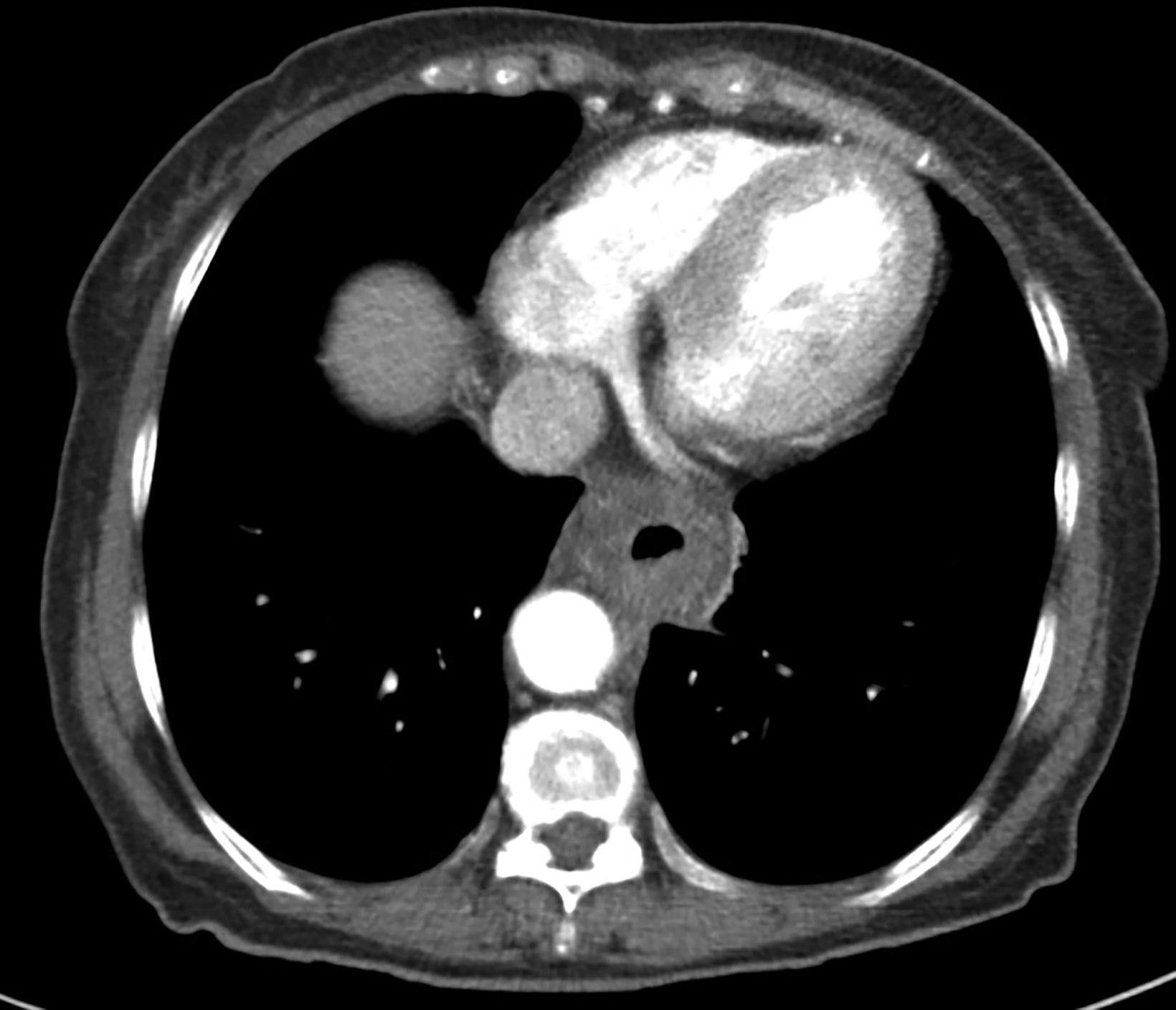
A 62-year-old woman was referred to our center for a moderately differentiated fungating esophageal adenocarcinoma at 25 cm from the incisors. A computed tomography (CT) scan (Figure 1) showed a mid to lower esophageal tumor with radial extension into to the pericardium and distally into the cardioesophageal junction (COJ) but no regional nodes or distant metastasis (TNM7 cT4 N0 M0). The patient was assessed by an upper gastrointestinal surgeon, who deemed the disease was inoperable. Treatment options were discussed, including upfront high-dose-rate brachytherapy (HDRBT) followed by definitive radiation therapy (dRT) or concurrent chemoradiotherapy (CRT). The patient understood and consented to the proposed treatment regimen consisting of 3 fractions of HDRBT followed by CRT to a dose of 50.4 Gy in 28 fractions.
HDRBT Procedure
Brachytherapy Applicator Insertion
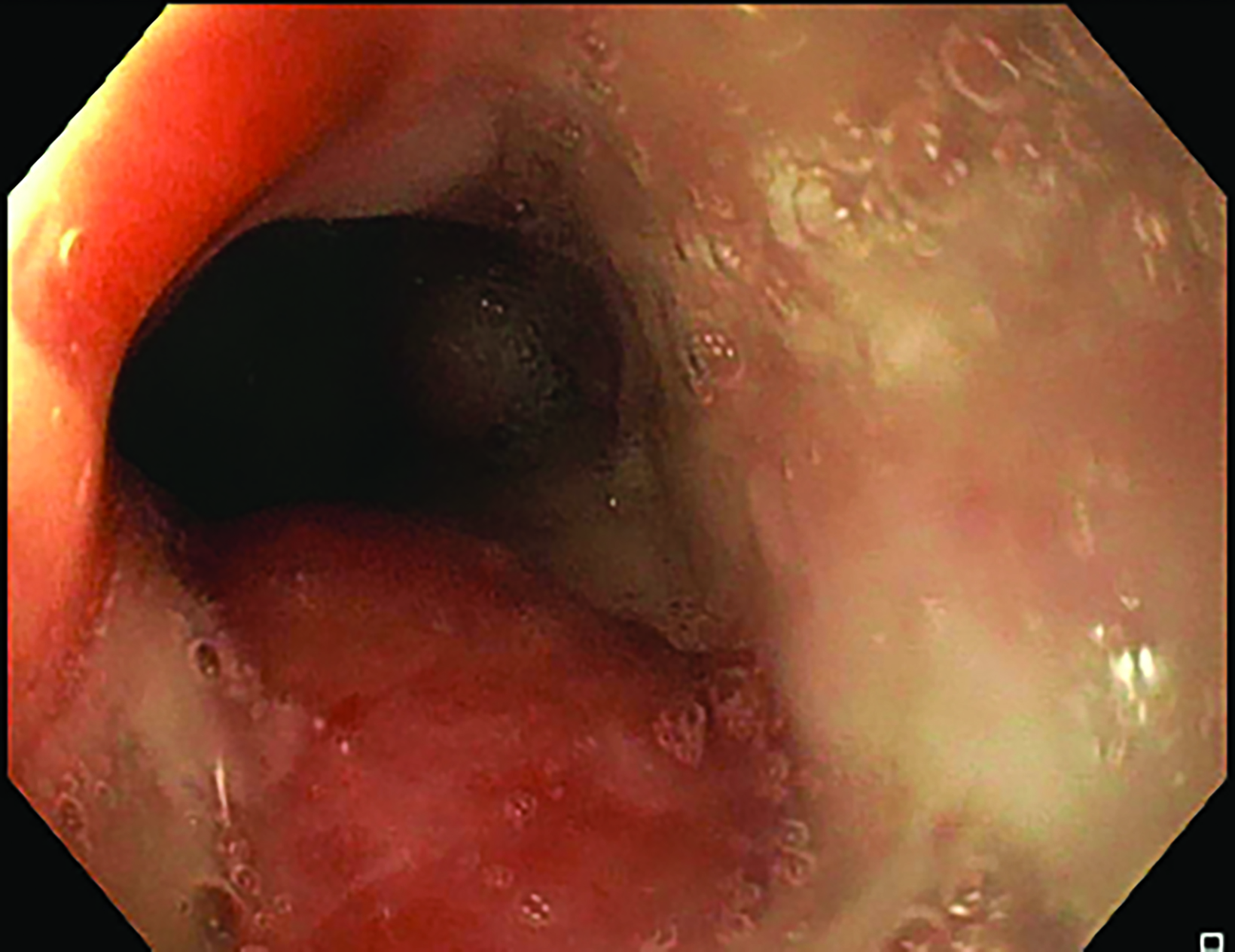
The insertion was completed under sedation in the operation theater with endoscopic and fluoroscopic guidance. During the first insertion, there was a circumferential tumor at 24 cm from the incisor with near complete obstruction of the lumen, needing balloon dilatation (Figure 2). Using endoscopy with fluoroscopic guidance, a radio-opaque marker was placed on the chest wall corresponding to the superior and inferior extension of the tumor. Following marker placement, a 6-mm diameter Nucletron intraluminal brachytherapy applicator was inserted with the distal part into stomach, also under fluoroscopic guidance. The brachytherapy applicator was anchored at the mouth with a bite block. For the second and third sessions, no balloon dilatation was needed due to good tumor response.
Treatment Planning
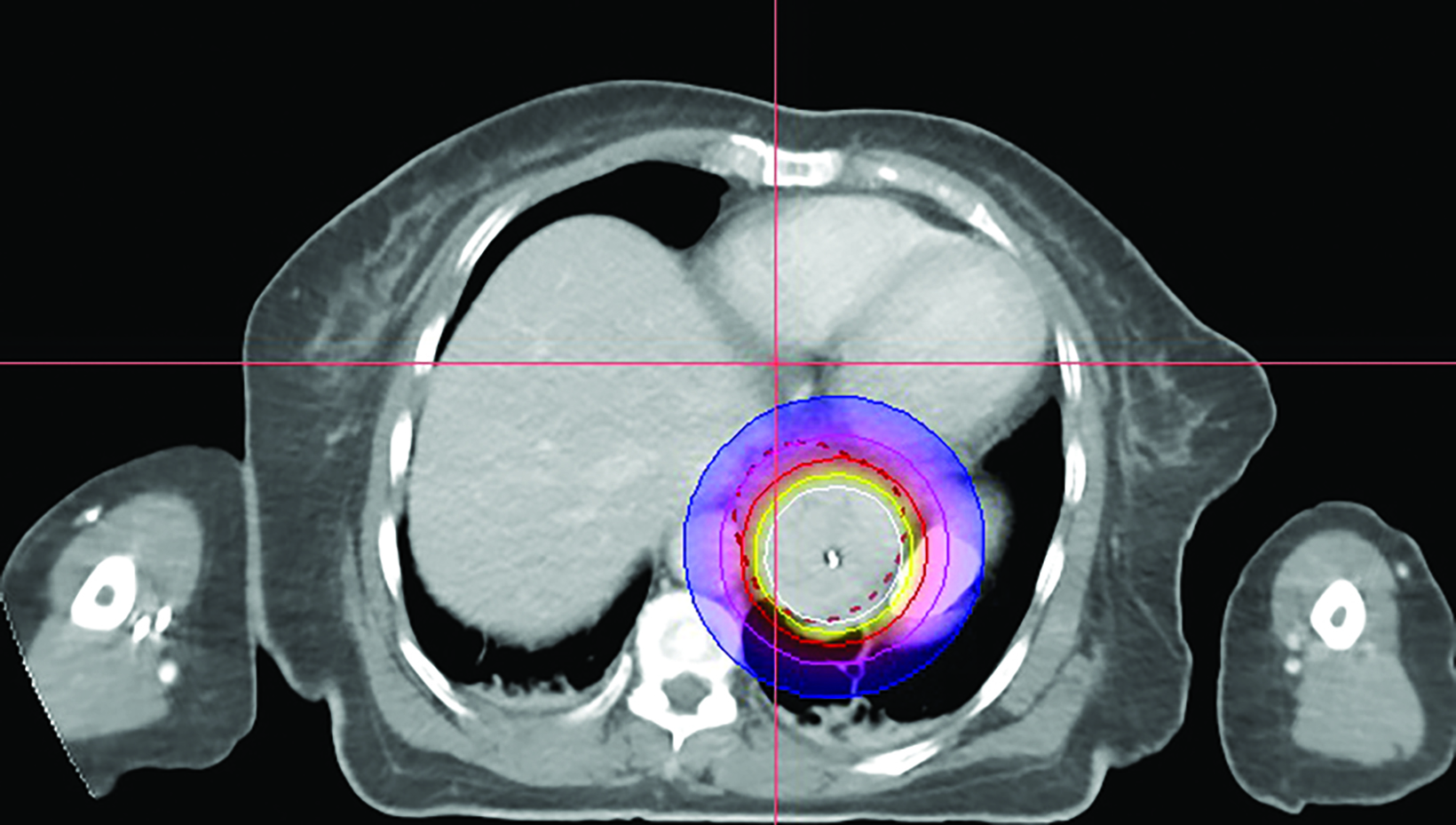
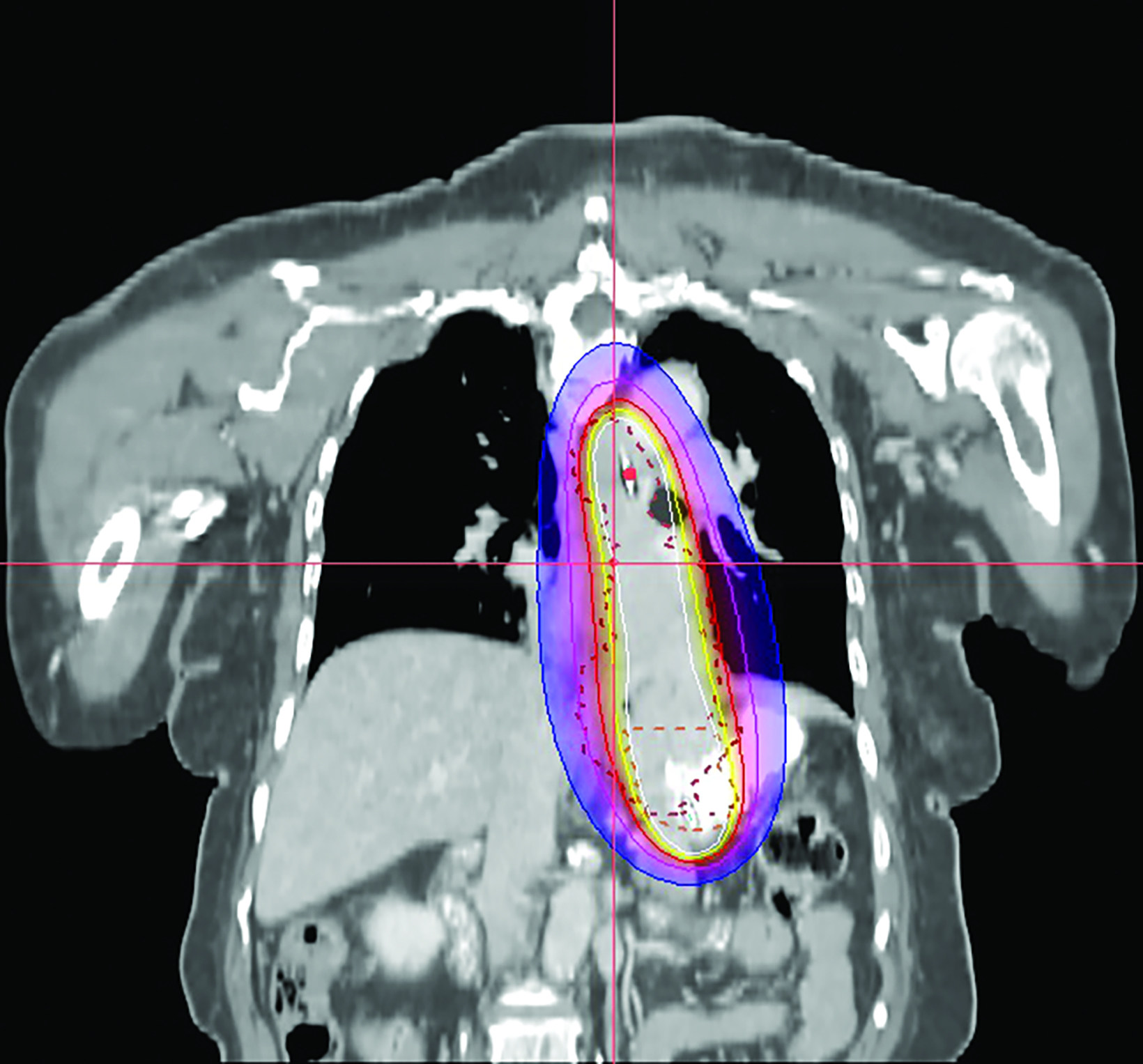
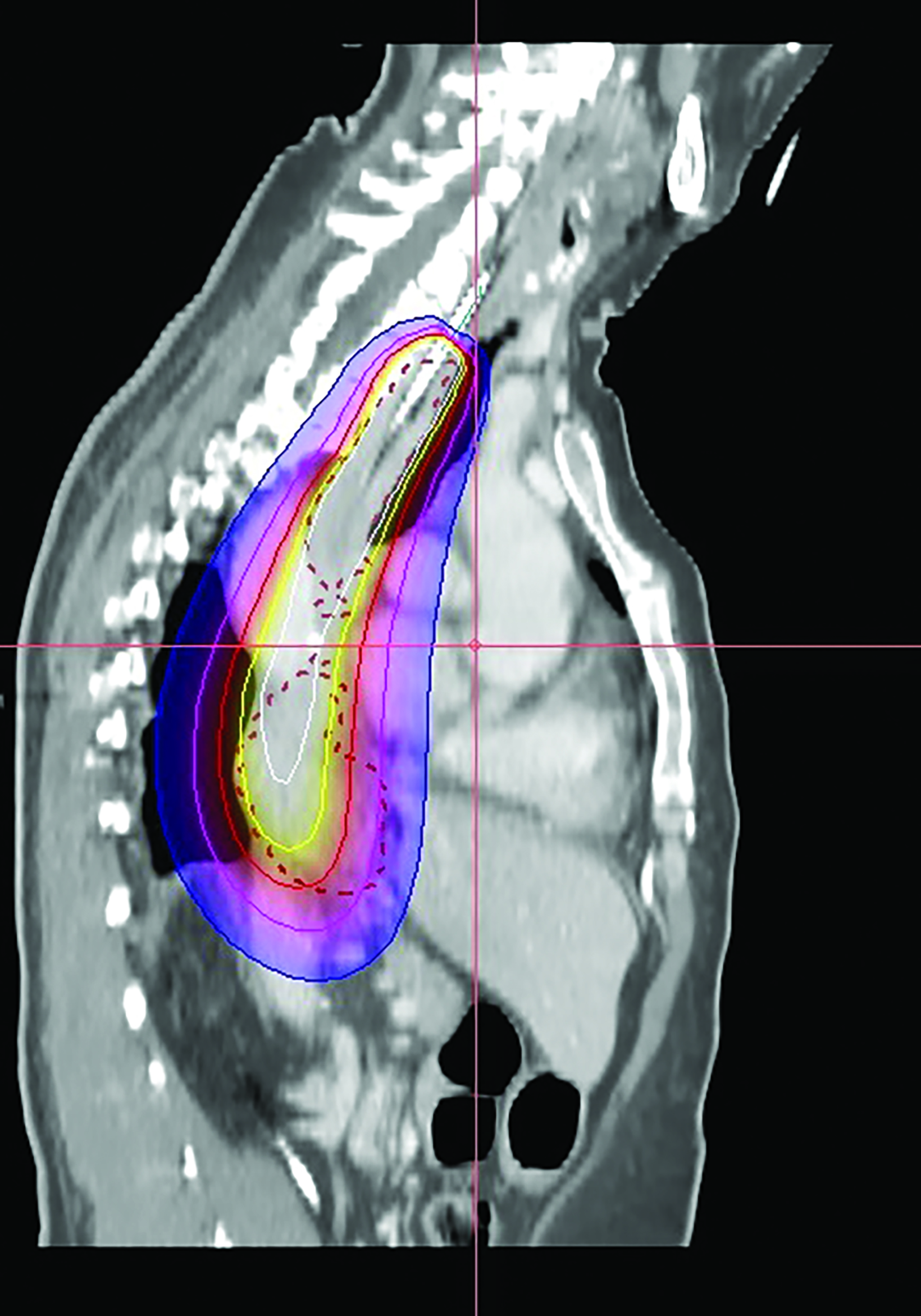
In the 2-mm-slice CT-simulation images, the contrast-enhancing tumor was marked as the gross tumor volume (GTV) with the superior and inferior extension corresponding to the ball bearing that was placed on the chest wall. A dose of 7 Gy was prescribed to cover the GTV plus a 1-cm safety margin superiorly and inferiorly using the Oncentra Masterplan V5.0 brachytherapy treatment planning system. Thereafter, the isodoses were normalized and adjusted graphically to have the 7 Gy isodose line covering the GTV, and heart dose constrained to the 6 Gy isodose line. The radial distance of the 7 Gy isodose is limited to 7 mm from the center of the applicator at the safety margin region superiorly and inferiorly, while allowing a more generous coverage at thicker tumor depth (Figures 3A-C). Maximum esophageal surface dose is 25 Gy to 52.88 Gy.
Treatment
The patient was treated with the Nucletron HDRBT afterloader system using an iridium-192 brachytherapy source with an initial source strength of 10 mCi. She was treated with 3 fractions of weekly 7 Gy HDRBT using the same plan. The intraluminal applicator was removed immediately after each session.
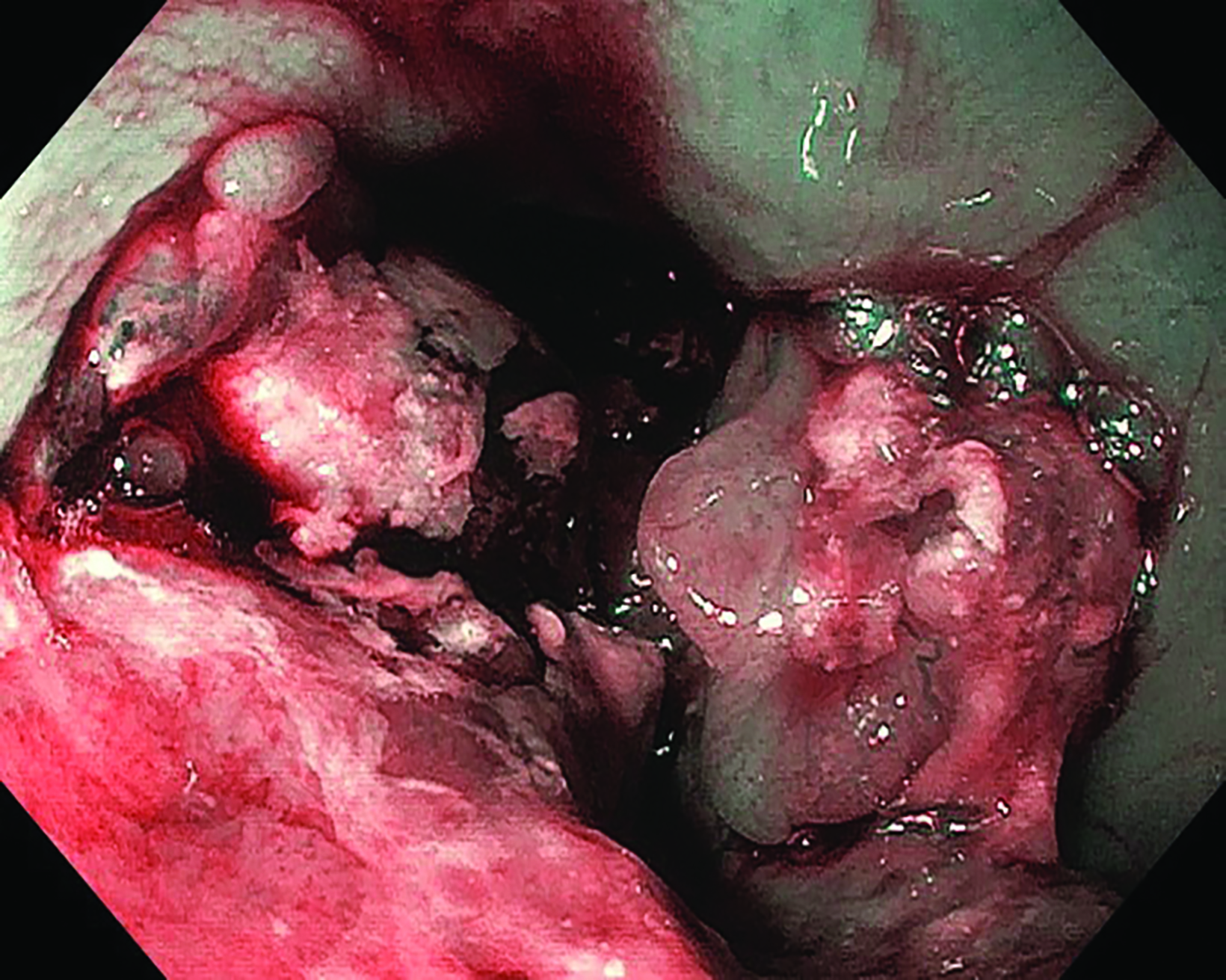
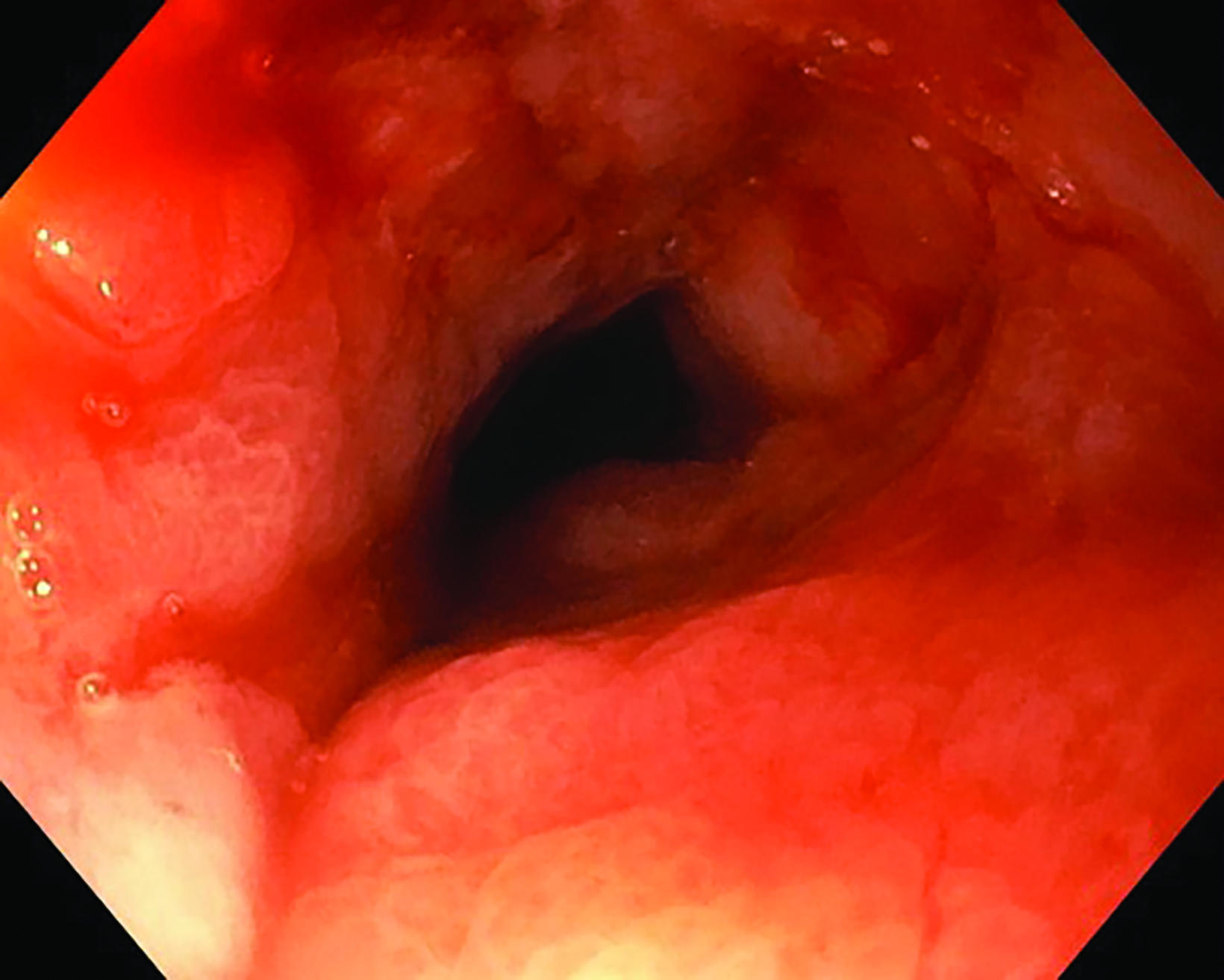
Following the second fraction of HDRBT, the patient had marked improvement in the dysphagia score. She was tolerating a semi-solid diet and her weight was progressively increasing. During the third HDRBT applicator insertion, esophagogastroduodenoscopy showed gross resolution of the intraluminal esophageal tumor (Figure 4).
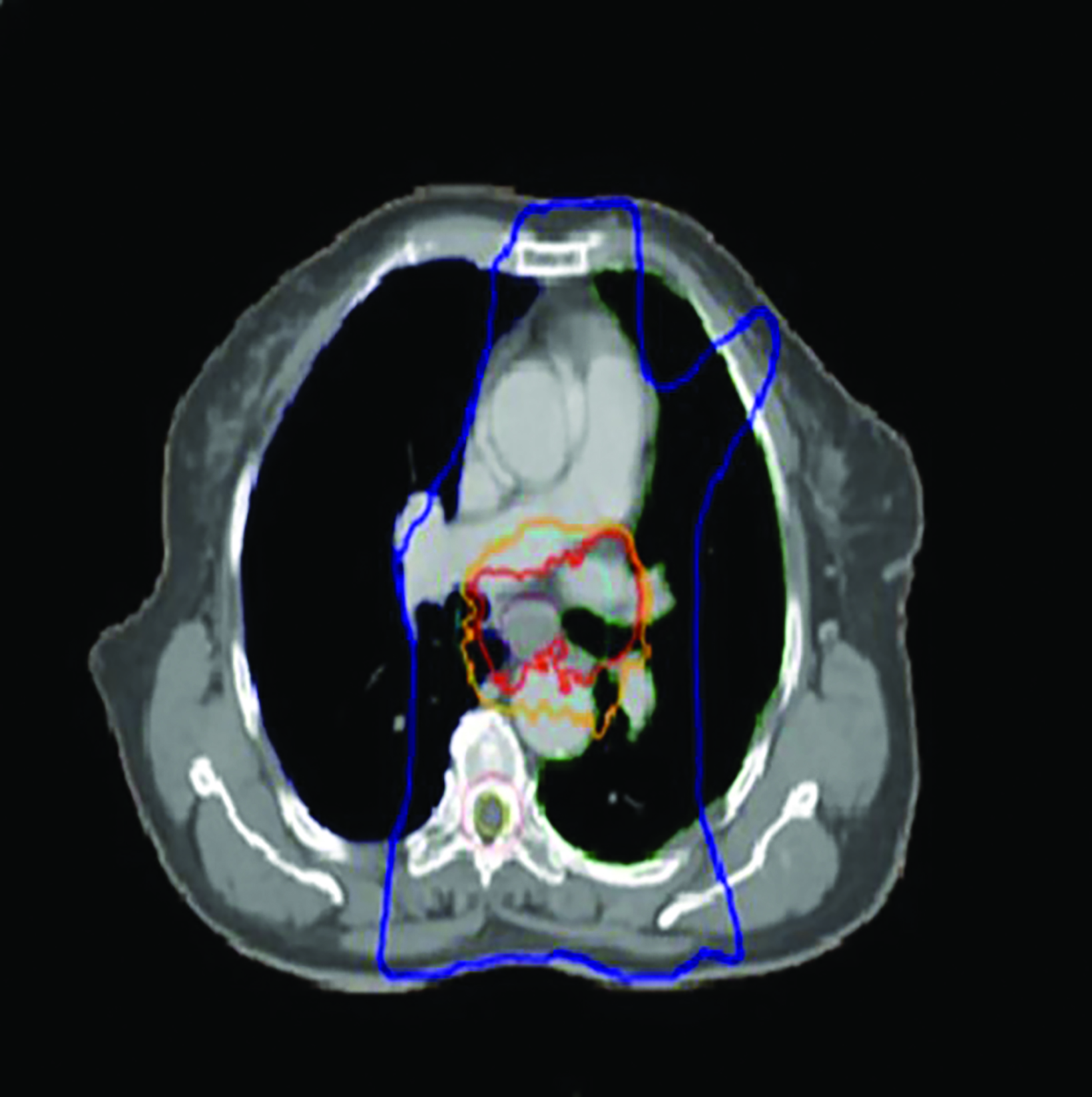
After completing HDRBT treatment, the patient was subjected to external-beam radiation therapy (EBRT) using IMRT to a total dose of 50.4 Gy in 28 fractions over 5 weeks with concurrent oral capecitabine 1g twice a day on radiation therapy days only. The treatment volume encompassed a 4-cm superior and 4-cm inferior margin, which extend into the fundus of the stomach; a 1-cm radial margin around the GTV to generate the clinical target volume (CTV); and a 1-cm margin was added for the planning target volume (PTV) (Figure 5) as per standard contouring guidelines. No elective nodes were included. All organs at risk such as bilateral lung, heart, spinal cord, bilateral kidney, and liver were defined in each CT slice and met the normal tolerance as per QUANTEC. Daily electronic portal imaging and weekly cone-beam CT were used for treatment verification. Oral capecitabine was used as the patient refused in- travenous chemotherapy. On completion of CRT, the patient had grade 1 esophagitis but was still tolerating well orally. She was prescribed a proton pump inhibitor for 6 months.
Follow-up
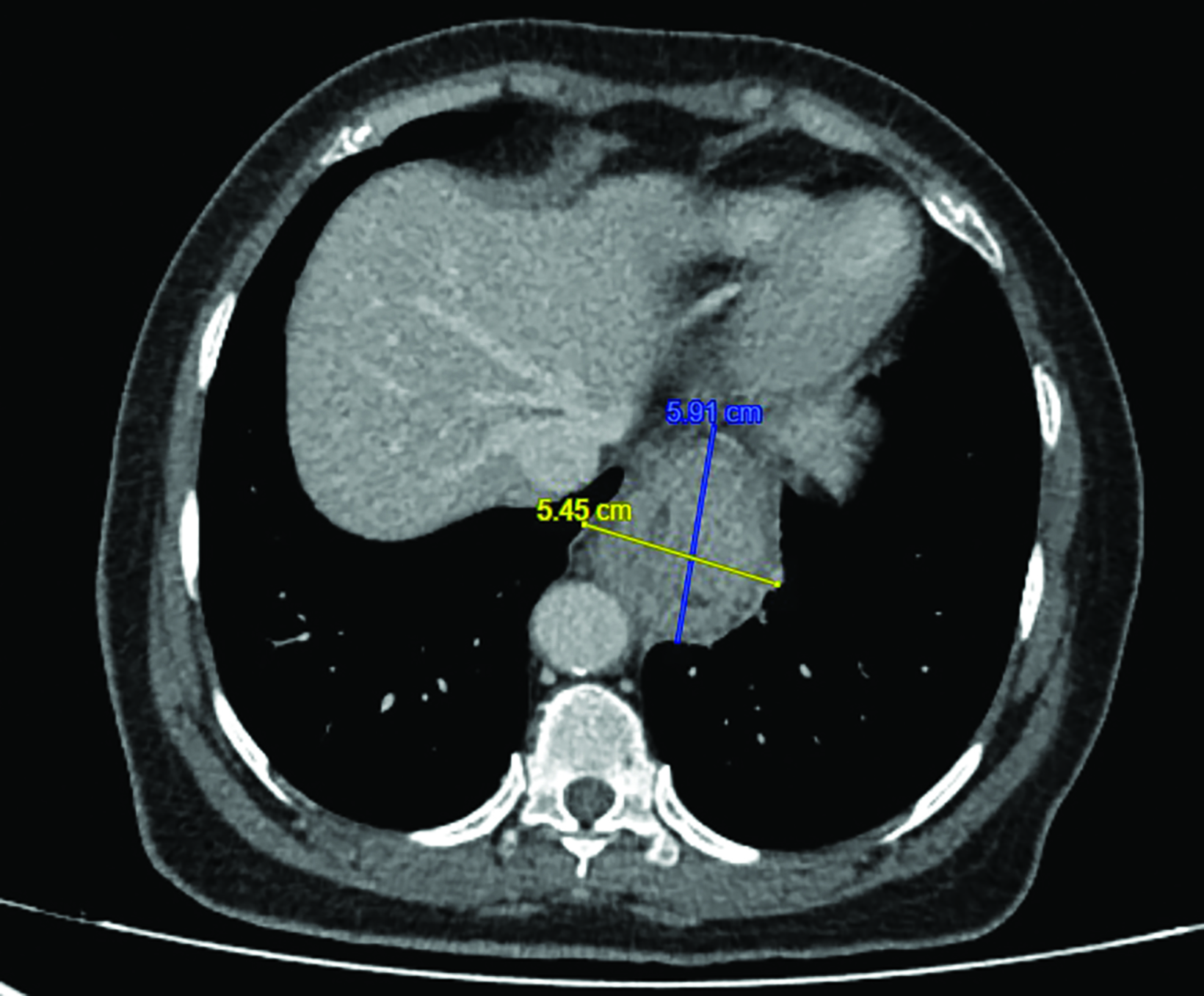
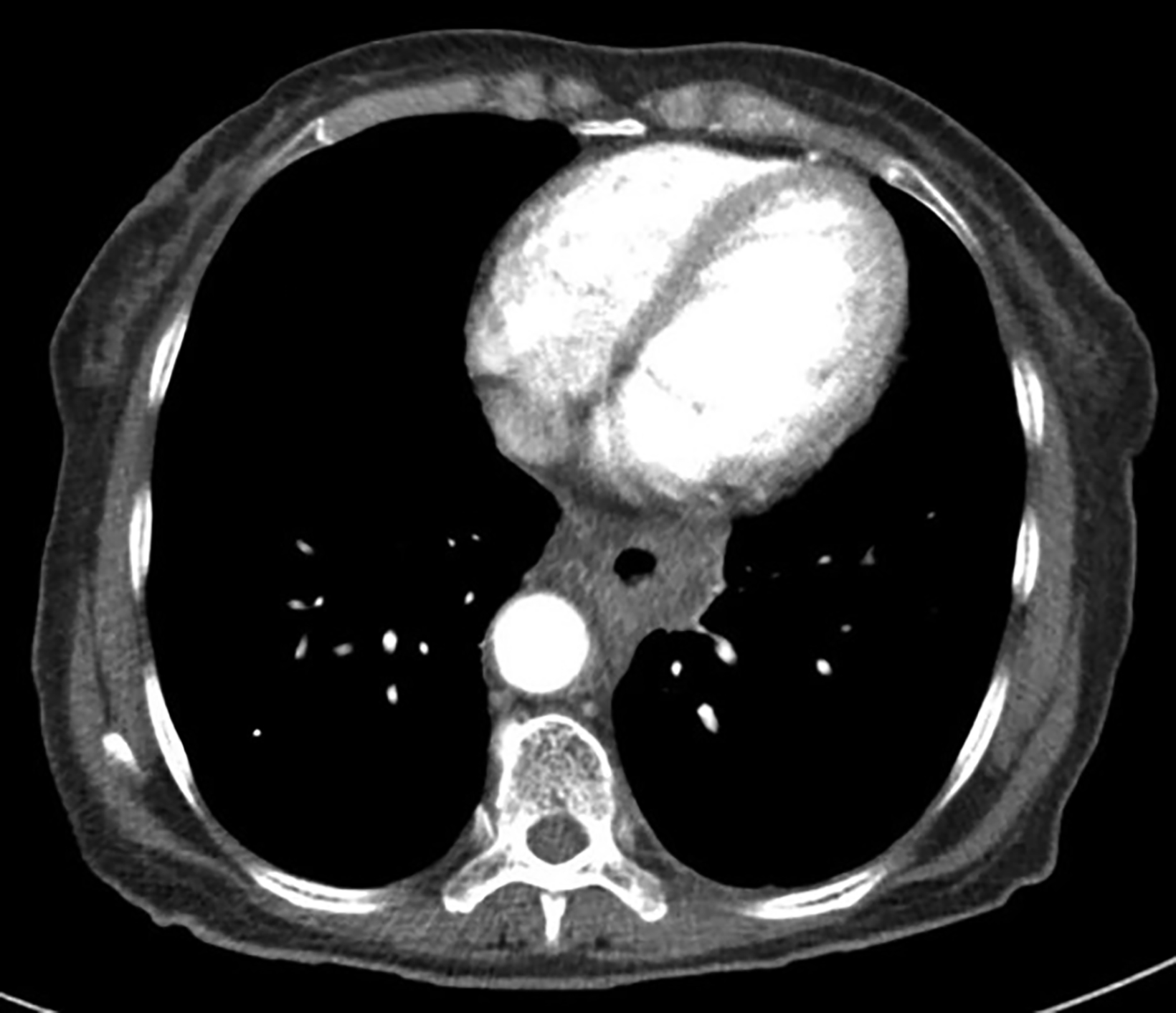
Endoscopic examination after 3 months showed complete clinical response with fibrosis and mild stricture at the gastroesophageal junction, which was easily dilated with a balloon (Figure 6A). CT-TAP (CT of the thorax, abdomen and pelvis) at 3 (Figures 6A-B) and 18 months (Figure 7) showed focal wall thickening along the irradiated esophagus with no evidence of recurrence.
The patient’s last physical follow-up with us was 12 months after completing treatment, at which time she refused endoscopy or imaging investigations. She was tolerating an oral diet with mild grade 1 dysphagia and had gained weight. Thereafter, we followed up with her via 2 monthly phone calls as she declined any assessment and reported a good quality of life. During phone assessments at 29, 34 and 40 months, she reported grade 1 dysphagia and was otherwise well. Unfortunately, at 48 months following treatment, we were informed by a family member that the patient had died 1 week earlier, with a likely diagnosis of a cerebrovascular accident.
Discussion
Based on the RTOG 85-01 intergroup trial and RTOG 94-05, radiation dose to 50 Gy with concurrent chemotherapy is the preferred treatment for patients with unresectable esophageal cancer.1,2 While CRT improves locoregional control, locoregional recurrence (LRR) still affects about 50% of patients, half of whom have an isolated recurrence without distant metastases.3 Furthermore, the majority of LRRs occur at the primary site vs being nodal, suggesting a benefit from dose escalation to the primary tumor.3 Radiation therapy dose escalation has been reported to improve the outcomes in a few studies involving unresectable esophageal cancer patients.2,4 The ARTDECO dose escalation trial in esophageal cancer patients showed a numerically superior 3-year, local progression-free survival (LPFS) in the dose-escalated (61.6 Gy) arm at 73% vs 70% in the standard (50.4 Gy) arm, although it did not reach statistical significance. This study, which used advanced radiation therapy techniques, had better pretreatment staging investigations and a different choice of concurrent chemotherapy regimen compared with earlier studies that showed increased acute and late toxicity with dose escalation.5
Intraluminal HDRBT of the esophagus has the distinct advantage of delivering a high dose of radiation to the tumor while sparing the normal organs. However, use of HDRBT is riddled with significant toxicity, such as esophageal fistula and stricture.6-9 American Brachytherapy Society recommendations for esophageal HDRBT include use of an external applicator whose diameter is 0.6 to 1.0 cm, EBRT delivered before HDRBT, and maximum tumor length under 10 cm.10 The initial rationale for the larger applicator size was to reduce the mucosal dose in relation to dose at the prescription point. However, this is an old guideline and has since been removed from the ABS website. Newer studies have included tumors larger than 10 cm in length, and some have delivered HDRBT before commencing EBRT.6,10
We theorize that the addition of HDRBT to EBRT in esophageal cancer improves the local control rate and provides durable alleviation of dysphagia.11,12 One randomized controlled trial even showed the superiority of HDRBT over EBRT boost.13 In most literature, an HDRBT boost was added following EBRT, not prior to it. While an HDRBT boost is usually delivered after EBRT, in 1 single-arm phase 2 study, HDRBT before EBRT is shown to be safe and effective in inducing rapid and durable relief from dysphagia prior to commencing curative intent CRT.13-16 Delivering HDRBT upfront also has the advantage of providing easy tumor identification and defining the target volumes as opposed to doing so after EBRT, which could significantly shrink the esophageal tumor. In addition, by starting with HDRBT, we could better recognize the tumor location compared with post-EBRT when usually no tumor is visible. Additional advantages are that with rapid significant tumor shrinkage, patients tend to have immediate improvement in their dysphagia score and nutrition status, and tend to be more receptive to subsequent proposed treatment with CRT.16
One study reported that applicator size may not correlate with the esophageal fistula rate.4 However, the dose per fraction of HDRBT showed a strong correlation with the fistula rates.17 In a study by Vuong et al, with the use of lower-dose fractionated HDRBT, there was only 1.2% fistula rates for all 70 patients. This study also shortened the overall treatment time by delivering HDRBT twice a week to a total dose of 20 Gy in 5 fractions and commencing EBRT the week immediately following HDRBT completion.4 When overall treatment time is below 8 weeks, studies have shown fewer local recurrences.18
In this patient, HDRBT was delivered over 3 weekly fractions of 7 Gy each to a total dose of 21 Gy. This dose schedule was chosen as the patient refused a more fractionated schedule of 20 Gy in 5 fractions treating 2 fractions a week due to logistical reasons. In the treatment planning process, we applied a 3D planning method with dose manipulation similar to dose painting in the IMRT. Rather than apply the dose prescription to a point at a radial distance from the center of the applicator, which produces a uniform cylindrical target, we ensured that 90% of the GTV received the prescribed dose of 7 Gy. The 7 Gy isodose curve was manipulated in such a way that generous coverage was allowed to areas with thicker tumor depth, while the same isodose line was restricted at smaller tumor depth or the safety margin region. This method of dose painting was described by Lettmaier et al who suggested that the use of 3DCT-based treatment planning has the advantage of dose manipulation over an applicator-based approach, which ignores surrounding normal structures.19
Conclusion
Use of an upfront 3D HDRBT boost with dose manipulation followed by IMRT showed good tumor response and minimal toxicity in this patient. Upfront HDRBT has the advantage of rapid relieving dysphagia, making the patient more receptive to subsequent treatment with concurrent CRT. Dose escalation with the aim to improve local control rate is feasible with HDRBT. Treatment for postradiation stricture, a known toxicity of radiation therapy/HDRBT, can be addressed by endoscopic balloon dilatation.
References
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627.
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167-1174.
- Versteijne E, van Laarhoven HWM, van Hooft JE, et al. Definitive chemoradiation for patients with inoperable and/or unresectable esophageal cancer: locoregional recurrence pattern. Dis Esophagus. 2015;28:453-459.
- Vuong T, Szego P, David M, et al. The safety and usefulness of high-dose-rate endoluminal brachytherapy as a boost in the treatment of patients with esophageal cancer with external beam radiation with or without chemotherapy. Int J Radiat Oncol Biol Phys. 2005;63(3):758-764.
- Hulshof MCCM, Geijsen D, Rozema T, et al. A randomized controlled phase III multicenter study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer: ARTDECO study. J Clin Oncol. 2020 38:4_suppl, 281-281.
- Taal BG, Aleman BM, Koning CC, Boot H. High dose rate brachytherapy before external beam irradiation in inoperable oesophageal cancer. Br J Cancer. 1996;74(9):1452-1457.
- Gaspar LE, Winter K, Kocha WI, Coia LR, Herskovic A, Graham M. A phase I/II study of external beam radiation, brachytherapy, and concurrent chemother- apy for patients with localized carcinoma of the esophagus (Radiation Therapy Oncology Group Study 9207): final report. Cancer. 2000;88(5):988-995.
- Schraube P, Fritz P, Wannenmacher MF. Combined endoluminal and external irradiation of inoperable oesophageal carcinoma. Radiother Oncol. 1997;44(1):45-51.
- Sharma V, Agarwal J, Dinshaw K, et al. Late esophageal toxicity using a combination of external beam radiation, intraluminal brachytherapy and 5-fluorouracil infusion in carcinoma of the esophagus. Dis Esophagus. 2000;13(3):219-225.
- Gaspar LE, Nag S, Herskovic A, Mantravadi R, Speiser B. American Brachytherapy Society (ABS) consensus guidelines for brachytherapy of esophageal cancer. Clinical Research Committee, American Brachytherapy Society, Philadelphia, PA. Int J Radiat Oncol Biol Phys. 1997;38(1):127-132.
- Hujala K, Sipilä J, Minn H, et al. Combined external and intra luminal radiotherapy in the treatment of advanced oesophageal cancer. Radiother Oncol. 2002;64:41-45.
- Rosenblatt E, Jones G, Sur RK, et al. Adding external beam to intra-luminal brachytherapy improves palliation in obstructive squamous cell oesophageal cancer: a prospective multi-centre randomized trial of the International Atomic Energy Agency. Radiother Oncol. 2010;97:488-494.
- Sur RK, Singh DP, Sharma SC, et al. Radiation therapy of esophageal cancer: role of high dose rate brachytherapy. Int J Radiat Oncol Biol Phys. 1992;22:1043-1046.
- Petrovich Z, Langholz B, Formenti S et al. Management of carcinoma of the esophagus: the role of radiotherapy. Am J Clin Oncol. 1991;14:80-86.
- Hyden EC, Langholz B, Tilden T et al. External beam and intraluminal radiotherapy in the treatment of carcinoma of the esophagus. J Thorac Cardiovasc Surg. 1988;96:237-241.
- Safaei AM, Ghalehtaki R, Khanjani N, et al. High-dose-rate intraluminal brachytherapy prior to external radiochemotherapy in locally advanced esophageal cancer: preliminary results. J Contemp Brachy. 2017;9(1):30-35.
- Muijs CT, Beukema JC, Mul VE, Plukker JT, Sijtsema NM, Langendijk JA. External beam radiotherapy combined with intraluminal brachytherapy in esophageal carcinoma. Radiother Oncol. 2012;102(2):303-308.
- Nishimura Y, Ono K, et al. Esophageal cancer treated with radiotherapy: impact of total treatment time and fractionation. Int J Radiat Oncol Biol Phys.1994;30(5):1099-1105.
- Lettmaier S, Strnad V. Intraluminal brachytherapy in oesophageal cancer: defining its role and introducing the technique. J Contemp Brachy. 2014;6(2):236-241.
Citation
GK A, SA S, H H, LV M, N M, MZA A. High-Dose-Rate Brachytherapy (HDRBT) Followed by Concurrent Chemoradiotherapy for Esophageal Adenocarcinoma. Appl Radiat Oncol. 2022;(4):34-40.
December 23, 2022