Multimodality management of colorectal liver oligometastases
SA-CME credits are available for this article here.
Colorectal cancer (CRC) represents the second-leading cause of cancer-related mortality in the United States.1 Due to drainage of the majority of intestinal mesentery through the hepatic portal venous system, metastases to the liver are the most common site of spread of CRC. While many patients present with widespread or extra-hepatic metastases, a subset of patients with limited liver metastases (LM) have been reported to experience high rates of long-term survival, and even cure, after liver resection.2-5 In a series of patients with CRC from the British Columbia Cancer Agency, 46% of those with metastatic disease presented with liver-only metastases, 38% of these had 1-3 sites of disease, and resected patients had the highest rates of survival.6 Patients with limited metastatic disease in which long-term control or even cure may be achievable are regarded as oligometastatic. The definition of oligometastatic is continually evolving, but typically refers to patients with 1-3, or 1-5 metastases.7 While larger-scale randomized trials are ongoing, exploratory studies have suggested the potential for improved survival with local therapy directed at oligometastatic CRC.8,9 Herein we review the treatment options for patients with oligometastatic CRC LM.
Treatment
Intent and Sequencing
A critical first step in managing patients with oligometastatic CRC is to define the intent of treatment: determining those patients who may have a chance for long-term control or cure and those whose quality of life would best be served by palliation. Either approach requires a multidisciplinary approach to sequence management of the primary tumor, metastases, and chemotherapy. Considerations include age, comorbidities, performance status, presence of symptoms from the primary tumor, extent and distribution of disease, and resectability of metastatic lesions. If the primary tumor is symptomatic, it is typically addressed first to limit risk of complications. Patients with asymptomatic primary tumor and limited LM may undergo simultaneous resection followed by adjuvant chemotherapy. Patients with asymptomatic primary tumor and extensive LM require systemic therapy first. Multi-agent fluorouracil (5-FU) based chemotherapy is first line; however, oxaliplatin (FOLFOX) and irinotecan (FOLFIRI) regimens may increase the risk of steatohepatitis and noncirrhotic portal hypertension when given preoperatively.10,11 A randomized trial of perioperative chemotherapy vs surgery alone demonstrated increased perioperative complications in the chemotherapy group (25% vs 16%, p = 0.04); however, there was no difference in operative mortality, and perioperative chemotherapy was associated with a sustained benefit in progression-free survival (PFS) (median 20.9 months vs 12.5 months for eligible patients, p = 0.035).12 Although there is no proven impact on overall survival (OS), many centers favor neoadjuvant chemotherapy as an assessment of biologic behavior and in vivo chemosensitivity, improving surgical selection and PFS. While randomized trials do not demonstrate an OS benefit to adjuvant chemotherapy after hepatic resection, patients with LM are likely to have unresected micrometastatic disease. Therefore, society guidelines, including the National Comprehensive Cancer Network, suggest 6 months of FOLFOX-based chemotherapy.13
Chemotherapy
While there is no universally accepted first-line chemotherapy regimen, the 5-FU-based multi-agent regimens (FOLFOX and FOLFIRI) achieve the most promising response rates in chemotherapy-naive patients.14 The administration of these regimens depends on patient tolerability and frequently require dose reductions and/or early discontinuation secondary to toxicity.
The addition of bevacizumab to 5-FU-based multi-agent chemotherapy demonstrates modest improvement in OS and PFS across all clinically relevant subgroups, albeit at the cost of a 10% absolute increase in grade ≥ 3 toxicity.15 Cetuximab or panitumumab may be added for patients with left-sided tumors that are RAS and BRAF wild-type, but should not be combined with bevacizumab. CRC is not generally sensitive to immunotherapy; however, patients with mutations in DNA mismatch repair genes (< 5% of all metastatic CRCs) have exhibited high response rates to pembrolizumab and nivolimab with or without ipilimumab.16
For patients who do not progress, the optimal duration of first-line chemotherapy is not established. Several randomized trials have compared pre-defined regimens vs maintenance chemotherapy, and none have demonstrated a clear difference in survival. These trials are heterogeneous, varying in the re-introduction of active agents at time of progression, which limits the ability to reach definitive conclusions.17-20 Importantly, the data clearly demonstrate that 5-FU, irinotecan, and oxaliplatin are the most active chemotherapy agents and should be given to all patients, whether it be concurrently or sequentially, and with or without maintenance. These regimens appear to have lower rates of cross-resistance, and patients offered all available agents during their disease course appear to have improved outcomes.21
The genetic profile of CRC is an important characteristic established at diagnosis, but there can be a discordance of genetic expression between the primary tumor and metastatic sites. In a novel gene expression analysis of both primary and metastatic CRC, investigators demonstrated that molecular signals that stratify for outcome in primary CRC were of limited prognostic utility in the subset of patients with resected CRC LM. Based on surgical pathology, investigators identified molecular signals risk stratifying patients into low-, intermediate-, and high-risk groups with relative 10-year OS rates of 94%, 45%, and 19%, respectively.22 Similarly, in a broader group of patients with CRC metastases to multiple sites, a multigene radiation sensitivity index score (RSI) suggested slightly greater radiation resistance in metastatic sites compared to CRC primary tumors, with the highest median resistance scores found in ovary, abdominal, and liver metastases.23 Molecular risk profiling of both primary and metastatic sites, in combination with other known prognostic features, likely reflects the future of individualized therapy.
To summarize the current systemic therapy guidelines, a 5-FU-based, multi-agent therapy remains the backbone of treatment for patients with metastatic CRC, regardless of the mutational status or metastatic burden. Newly diagnosed patients should be started on FOLFOX or FOLFIRI, with the addition of a monoclonal antibody based on mutational status. Given low rates of cross-resistance, patients should be offered second-line chemotherapy at progression. For the small percentage of patients with DNA mismatch repair mutations, treatment with immunotherapy is the preferred second-line treatment after progression on standard first-line therapy.
Surgical Resection
Surgical resection is the standard of care for low-volume CRC LM. Surgery has the largest body of evidence demonstrating long-term survival, although only 20% to 30% of metastatic patients are resectable at diagnosis.2-5 A limited group of patients, unresectable at presentation, may become surgical candidates following a favorable response to chemotherapy. This is known as down-staging, and patients who undergo resection after down-staging have similar outcomes to patients who are resectable at diagnosis.24 In select cases, surgical resection for bilateral liver involvement can be achieved with partial right and left hepatectomies. During surgical planning, if the expected liver remnant is not expected to be of sufficient volume, portal vein embolization may be performed. Embolization should prompt hypertrophy of the perfused liver and, if the remaining liver grows to an adequate volume, resection is feasible.25
Overall, for the high-performing patient with low-volume CRC LM, the standard of care is surgical resection of LM. This can be preceded by chemotherapy and/or portal vein embolization in cases appropriate for down-staging. For most patients who are not surgical candidates, other liver-directed therapies have emerged.
Radiofrequency and Cryoablation
For unresectable CRC LM, minimally invasive ablation techniques for LM treatment include radiofrequency ablation (RFA), microwave ablation, and cryoablation. The prospective EORTC 4004 phase II trial randomized 119 patients with unresectable CRC LM (< 10 LM and no extrahepatic disease) to systemic therapy alone (FOLFOX and bevacizumab) vs systemic therapy and RFA (+/- resection).9 The majority (73.3%) of patients had ≤ 5 metastases. After a median follow-up of 9.7 years, 10% of patients were alive in the systemic therapy arm and 35% in the combined modality arm. The combined modality arm demonstrated improved OS (HR 0.58, p = 0.01), median OS (45.6 months vs 40.5 months), and PFS (16.8 months vs 9.9 months, p = 0.005) compared to systemic therapy alone. Local progression occurred in 11 out of 170 RFA-treated lesions (6.5%), although the median tumor size was not reported. Postoperative complications included infection (13.3%), fever (16.7%), and hospitalization > 24 hours (13.3%). This study lends strong support to aggressive local control via ablation in CRC patients with limited LM. Contraindications to RFA are discussed in Table 1.
Ablation techniques should be considered when the number of peripheral LM is limited and preferably ≤ 3 cm. Tumors abutting or involving a bile duct should not be treated with ablation due to a high risk of biliary complications. Ablation technique selection is often institution and expertise dependent, but there are considerations for each. In thermal ablation, microwave is a newer technology than RFA, tends to be faster, can treat a larger area, and is less sensitive to the “heat sink” effect. This means microwave ablation will be more effective than RFA for larger tumors or those abutting vasculature. Cryoablation is more time intensive than thermal techniques, but visualization of the “ice ball” during freezing permits close monitoring of the ablation zone, which is not possible with RFA or microwave ablation.
Chemoembolization and Radioembolization
LM receive most of their blood supply from the hepatic artery. Transarterial chemoembolization (TACE) delivers a high local concentration of chemotherapy while reducing blood supply to LM via direct embolization of the hepatic artery. Transarterial radioembolization (TARE) delivers radioactive microspheres filled with yttrium 90 (90Y). In both techniques, normal tissue is relatively spared because of preserved portal vein blood supply. A randomized trial compared FOLFIRI to FOLFIRI with TACE in 74 patients who had progressed on second- or third-line chemotherapy. The addition of TACE improved response, OS, and increased time to extra-hepatic progression compared with FOLFIRI.26 As one would not expect a systemic effect after TACE, explaining improved extra-hepatic control is challenging. It is unclear if this suggests a benefit to treating local disease, or potentially reflects an imbalance in this small randomized trial given unexpected differences in extra-hepatic progression.
Multiple randomized trials have demonstrated the safety and efficacy of90Y with systemic therapy in patients with LM. A phase III trial compared 5-FU alone vs 90Y with 5-FU in unresectable, chemotherapy-refractory, liver-only metastases. Time to liver progression was improved (2.1 months vs 5.5 months, p = 0.003), but there was no difference in OS.27
Subsequently, 3 randomized phase III trials evaluated the addition of90Y to first-line chemotherapy for patients with liver-only or liver-dominant metastatic CRC. These studies, known as FOXFIRE, SIRFLOX, and FOXFIRE-Global, were published in a combined analysis of 1103 patients that randomized patients to FOLFOX alone vs a single dose of90Y during cycle 1 or 2 of FOLFOX. Median OS was not significantly different (23.9 months vs 23.4 months, p = 0.61), and there was no difference in OS for the prespecified analysis of patients with liver-only metastases.28 A post-hoc analysis of the SIRFLOX and FOXFIRE-Global trials evaluated right-sided vs left-sided primary tumors with the knowledge that right-sided primary tumors are a poor prognostic factor. Of the 739 patients in these 2 trials, 179 patients (24.2%) had right-sided primary tumors and improvement in median OS (17.1 months vs 22 months, p = 0.008) with the addition of90Y to FOLFOX. For patients with liver-only metastases, PFS improved for both right-sided (9.6 months vs 13.2 months, p = 0.001) and left-sided primary tumors (12.5 months vs 15.3 months, p = 0.015). The addition of90Y to first-line systemic therapy does not improve OS in an unselected group of patients with CRC LM, but future studies may reveal a benefit in a subgroup of patients.
TACE and TARE should be considered in patients with liver-dominant metastatic disease who progress on first- or second-line therapy or have residual LM after a favorable response to systemic therapy. They are preferred over ablation and SBRT when there are multiple LM in a lobe of the liver that can be treated simultaneously. The efficacy of TACE and TARE are similar, but TARE is more likely to promote liver remnant hypertrophy and should be favored if future resection is planned.29,30 TACE is associated with an increased incidence of post-embolization syndrome and hospitalization, and carries a high risk of hepatic decompensation when portal vein invasion is present. TARE can be used with limited portal vein thrombus as long as the portal vein invasion does not involve the main trunk; otherwise, SBRT is preferred.30
External-beam Radiation, Stereotactic Body Radiation Therapy, and Protons
The use of external-beam radiation for LM was historically limited by intolerance of the liver to high doses of radiation and subsequent risk of radiation-induced liver disease (RILD). As treatment delivery, image guidance, and motion management techniques have advanced, we can now deliver ablative doses of radiation while sparing normal liver.
For patients with CRC LM, stereotactic body radiation therapy (SBRT) has proven effective with reliable dosimetry and high rates of local control (LC), as outlined in Table 2. Low rates of toxicity have been reported, with the incidence of RILD from SBRT rarely described in noncirrhotic patients.31
A consensus SBRT dose for CRC LM does not exist; however, it is reported that higher BED (biologically equivalent dose) correlates with improved LC. In a retrospective multi-institutional series of 427 patients with 568 LM treated with SBRT, BED10≥100 Gy demonstrated improved 2-year LC of 77.2% vs 59.6% for BED10<100 Gy. OS was also improved with higher dose (27 months vs 15 months, p < 0.0001).36 Dose prescription varies significantly throughout published trials, but the lack of RILD suggests greater liver tolerance than previously proposed, and an opportunity for further dose escalation in noncirrhotic patients.
Tumor volume has been established as a driving factor, albeit variable, in the LC of lesions treated with SBRT, with lesions > 40 cm3 demonstrating decreased LC and OS.36 Most phase I and II studies required lesions < 6 cm for inclusion. Hong et al published the first series of LM treated with proton SBRT, and included larger tumors.37 For tumors ≥ 6 cm, they report LC of 73.9% and 65.2% at 1 and 3 years, respectively, challenging the theory that SBRT is not effective for large tumors. Furthermore, they also demonstrated the strongest predictor for inferior LC was not lesion size but mutational status. Tumors with a KRAS mutation demonstrated significantly decreased LC of 42.9% compared with 72.1% in tumors without the mutation (p = 0.02). The same has been reported in the90Y literature suggesting this as a marker of radiation resistance.41 Presence of a TP53 mutation was also associated with decreased LC of 46.2% vs 70.5% for wild type (p = 0.08), and tumors with both mutations had a 1-year LC of 20% vs 69.2% for all others (p = 0.001).37
In addition to following established SBRT constraints,42,43 (Table 3) many centers extrapolate from surgical literature estimating a minimum necessary residual volume of liver of 700 cm3, and spare this volume below an ablative dose-level. While this is a frequently applied estimate, the minimum required functional reserve likely varies based on many factors (age, BMI, liver size and health). Additional models for liver constraints use mean dose, effective volume of radiated liver (Veff),44 and functional imaging to spare more critical portions of a functioning liver.45 We recommend evaluating multiple toxicity models to balance the risk-benefit ratio for each patient. Although not uniformly agreed upon, dose constraints to the central liver are used to limit toxicity such as biliary stricture. The central liver is most commonly delineated as an expansion of the portal vein, and toxicity is more common in the treatment of primary biliary malignancy, such as cholangiocarcinoma. This suggests that in addition to radiation dose, disruption of normal biliary tree architecture likely contributes to the risk of central liver toxicity.46 The importance of dose constraints to the duodenum and small bowel come from the toxicity seen following the early pancreas SBRT experience and the increased incidence of ulceration, hemorrhage, and perforation. Generally, the experience of liver SBRT has been in heavily pretreated patients, who often have received prior liver-directed therapy and multiple lines of chemotherapy. Comparative analyses of SBRT with other liver-directed therapies are scarce, but those available demonstrate that SBRT patients commonly have less favorable characteristics, but equal or superior control rates. Jackson et al compared a cohort of SBRT patients with RFA and found a higher local failure risk with RFA for tumors ≥ 2 cm.47 Franzese et al performed a propensity-score-based comparison of SBRT with microwave ablation and showed a reduced risk of local relapse with SBRT (adjusted HR 0.31; p = 0.005).48 Shen et al also completed a propensity-score-based comparison of SBRT with TACE, for hepatocellular carcinoma lesions 3-8 cm, and found that SBRT demonstrated superior LC and OS, and was especially effective for recurrent cases.49 The favorable outcomes of SBRT are likely attributable to the high reliability of SBRT dosimetry, image guidance, and delivery of the planned treatment.
Conclusion
Oligometastatic CRC LM comprises a heterogeneous group of patients who require individual consideration when assessing treatment decisions. Multi-agent systemic therapy remains the backbone of treatment, but there are several options for complementary liver-directed therapy. LM location, size, and baseline hepatic function are the most important considerations when selecting optimal liver-directed therapy.
Surgical resection remains the standard of care, but the development of nonsurgical liver-directed therapies have been vital for the 70% of patients unresectable at diagnosis. Thermal and cryoablation techniques are best suited for LM ≤ 3 cm, of limited number, and in the periphery of the liver. SBRT is effective for small to medium LM and portal vein thrombosis, but should be carefully considered if multiple lesions require treatment. TACE or TARE is best for patients with higher volume LM in which treatment of a hepatic segment or lobe will address multiple LM synchronously. Table 1 summarizes indications and contraindications that can aid treatment selection based on individual patient and tumor factors.
Patients will commonly require more than 1 liver-directed therapy, and critical evaluation of disease response and toxicity to prior therapy should be considered. Given the complex clinical decision making and lack of definitive randomized evidence for CRC LM, multidisciplinary care by physicians specializing in the treatment of LM is imperative.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309-318; discussion 318-321.
- Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247(1):125-135.
- Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575-4580.
- Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13(5):668-676.
- Ksienski D, Woods R, Speers C, Kennecke H. Patterns of referral and resection among patients with liver-only metastatic colorectal cancer (MCRC). Ann Surg Oncol. 2010;17(12):3085-3093.
- Correa RJ, Salama JK, Milano MT, Palma DA. Stereotactic body radiotherapy for oligometastasis: opportunities for biology to guide clinical management. Cancer J. 2016;22(4):247-256.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185): 2051-2058.
- Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109(9).
- Robinson SM, Wilson CH, Burt AD, Manas DM, White SA. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19(13):4287-4299.
- Wiseman JT, Guzman-Pruneda F, Xourafas D, et al. Impact of neoadjuvant chemotherapy on the postoperative outcomes of patients undergoing liver resection for colorectal liver metastases: a population-based propensity-matched analysis. J Am Coll Surg. 2019.
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208-1215.
- National Comprehensive Cancer Network, NCCN Guidelines & Clinical Resources. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed June 12, 2019.
- Abrams TA, Meyer G, Schrag D, Meyerhardt JA, Moloney J, Fuchs CS. Chemotherapy usage patterns in a US-wide cohort of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2014;106(2):djt371.
- Hurwitz HI, Tebbutt NC, Kabbinavar F, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist. 2013;18(9):1004-1012.
- Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182-1191.
- Adams RA, Meade AM, Seymour MT, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011;12(7):642-653.
- Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol. 2009;27(34):5727-5733.
- de Gramont A, Buyse M, Abrahantes JC, et al. Reintroduction of oxaliplatin is associated with improved survival in advanced colorectal cancer. J Clin Oncol. 2007;25(22):3224-3229.
- Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol. 2006;24(3):394-400.
- Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22(7):1209-1214.
- Pitroda SP, Khodarev NN, Huang L, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun. 2018;9(1):1793.
- Ahmed KA, Fulp WJ, Berglund AE, et al. Differences between colon cancer primaries and metastases using a molecular assay for tumor radiation sensitivity suggest implications for potential oligometastatic SBRT patient selection. Int J Radiat Oncol Biol Phys. 2015;92(4):837-842.
- Maeda Y, Shinohara T, Nagatsu A, Futakawa N, Hamada T. Long-term outcomes of conversion hepatectomy for initially unresectable colorectal liver metastases. Ann Surg Oncol. 2016;23 Suppl 2:S242-248.
- Shindoh J, Tzeng CW, Aloia TA, et al. Portal vein embolization improves rate of resection of extensive colorectal liver metastases without worsening survival. Br J Surg. 2013;100(13):1777-1783.
- Fiorentini G, Aliberti C, Tilli M, et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 2012;32(4):1387-1395.
- Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28(23): 687-3694.
- Wasan HS, Gibbs P, Sharma NK, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18(9):1159-1171.
- Ahmadzadehfar H, Meyer C, Ezziddin S, et al. Hepatic volume changes induced by radioembolization with 90Y resin microspheres. A single-centre study. Euro J Nucl Med Mol Imaging. 2013;40(1):80-90.
- Kim DY, Han K-H. Transarterial chemoembolization versus transarterial radioembolization in hepatocellular carcinoma: optimization of selecting treatment modality. Hepatol Int. 2016;10(6):883-892.
- Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S94-100.
- Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45(7): 823-830.
- Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27(10):1572-1578.
- Mendez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase I-II study. Acta Oncol. 2006;45(7):831-837.
- Scorsetti M, Comito T, Clerici E, et al. Phase II trial on SBRT for unresectable liver metastases: long-term outcome and prognostic factors of survival after 5 years of follow-up. Radiat Oncol. 2018;13(1):234.
- Mahadevan A, Blanck O, Lanciano R, et al. Stereotactic body radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch(R) Patient Registry. Radiat Oncol. 2018;13(1):26.
- Hong TS, Wo JY, Borger DR, et al. Phase II study of proton-based stereotactic body radiation therapy for liver metastases: importance of tumor genotype. J Natl Cancer Inst. 2017;109(9).
- McPartlin A, Swaminath A, Wang R, et al. Long-term outcomes of phase 1 and 2 studies of SBRT for hepatic colorectal metastases. Int J Radiat Oncol Biol Phys. 2017;99(2):388-395.
- Sufficool DC, McGee P, Swenson S, et al. Proton SBRT for liver metastases - results of 5-year experience for 80 hepatic lesions based on NRG-BR001. Int J Radiat Oncol Biol Phys. 2018;102(3):S165-S166.
- Joo JH, Park JH, Kim JC, et al. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys. 2017;99(4):876-883.
- Lahti SJ, Xing M, Zhang D, Lee JJ, Magnetta MJ, Kim HS. KRAS status as an independent prognostic factor for survival after yttrium-90 radioembolization therapy for unresectable colorectal cancer liver metastases. J Vasc Interv Radiol. 2015;26(8):1102-1111.
- Pollom EL, Chin AL, Diehn M, Loo BW, Chang DT. Normal tissue constraints for abdominal and thoracic stereotactic body radiotherapy. semin radiat oncol. 2017;27(3):197-208.
- Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18(4):215-222.
- Ten Haken RK, Martel MK, Kessler ML, et al. Use of Veff and iso-NTCP in the implementation of dose escalation protocols. Int J Radiat Oncol Biol Phys. 1993;27(3):689-695.
- Tsegmed U, Kimura T, Nakashima T, et al. Functional image-guided stereotactic body radiation therapy planning for patients with hepatocellular carcinoma. Med Dosim. 2017;42(2):97-103.
- Osmundson EC, Wu Y, Luxton G, Bazan JG, Koong AC, Chang DT. Predictors of toxicity associated with stereotactic body radiation therapy to the central hepatobiliary tract. Int J Radiat Oncol Biol Phys. 2015;91(5):986-994.
- Jackson WC, Tao Y, Mendiratta-Lala M, et al. Comparison of stereotactic body radiation therapy and radiofrequency ablation in the treatment of intrahepatic metastases. Int J Radiat Oncol Biol Phys. 2018;100(4):950-958.
- Franzese C, Comito T, Clerici E, et al. Liver metastases from colorectal cancer: propensity score-based comparison of stereotactic body radiation therapy vs. microwave ablation. J Cancer Res Clin Oncol. 2018;144(9):1777-1783.
- Shen P-C, Chang W-C, Lo C-H, et al. Comparison of stereotactic body radiation therapy and transarterial chemoembolization for unresectable medium-sized hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2019; S0360-3016(19):30820.
- Gaba RC, Lokken RP, Hickey RM, et al. Quality improvement guidelines for transarterial chemoembolization and embolization of hepatic malignancy. J Vasc Interv Radiol. 2017;28(9):1210-1223.e1213.
- Benhaim L, El Hajjam M, Malafosse R, et al. Radiofrequency ablation for colorectal cancer liver metastases initially greater than 25 mm but downsized by neo-adjuvant chemotherapy is associated with increased rate of local tumor progression. HPB (Oxford). 2018;20(1):76-82.
- Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010;33(1):11-17.
- Kingham TP, Tanoue M, Eaton A, et al. Patterns of recurrence after ablation of colorectal cancer liver metastases. Ann Surg Oncol. 2012;19(3):834-841.
- van Duijnhoven FH, Jansen MC, Junggeburt JM, et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol. 2006;13(5):651-658.
- Veltri A, Sacchetto P, Tosetti I, Pagano E, Fava C, Gandini G. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol. 2008;31(5):948-956.
Case Example
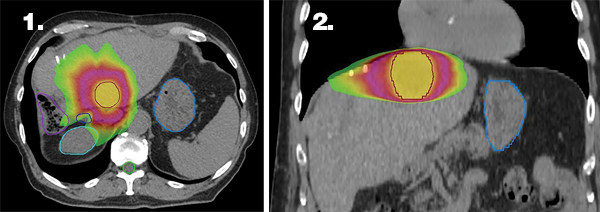
Figures 1 and 2 illustrate the highly conformal stereotactic body radiation therapy (SBRT) treatment plan with sharp dose fall-off near the critical structures (bile duct and bowel). An internal target volume (ITV-not shown) was generated by combining the gross tumor volume (GTV) from 3 separate breath-hold scans. The ITV was expanded by 5 mm uniformly to create a PTV, which is delineated by the red outline. The prescription dose of 54 Gy is represented by the yellow color wash, 27 Gy by the magenta color wash, and 15 Gy by the lime green color wash. Delineated organs at risk include the stomach in light blue, bile duct in dark blue, right kidney in teal, large bowel in purple, and spinal cord in lime green.
A 63-year-old man was diagnosed with cT2N1bM1a, stage IVA, adenocarcinoma (KRAS mutated) of the rectum with multiple liver metastases. The patient was initiated on chemotherapy with FOLFOX, and bevacizumab was added on cycle 2. After 3 cycles of chemotherapy the patient was treated with90Y to the right liver, the dominant site of liver metastases. Following 4 additional cycles of chemotherapy, computed tomography (CT) imaging of the chest, abdomen, and pelvis demonstrated a favorable response to therapy with a reduction in size of the primary rectal mass, near resolution of regional lymph nodes, and significant volume reduction of liver metastases. The patient’s CEA had decreased from 1205 ng/mL to 13 ng/mL during this time and he was continued on chemotherapy for 3 cycles (bevacizumab held the last cycle in anticipation of possible surgery). Follow-up imaging demonstrated a continued response to chemotherapy, so the patient was taken for surgical resection of the primary tumor via laparoscopic transanal total mesorectal excision. Surgical pathology demonstrated 0.5 cm of invasive moderately differentiated adenocarcinoma with a partial response to treatment. There was a microscopic focus of residual disease in only 1 of 27 lymph nodes, final pathologic staging ypT1N1a(mic). Six weeks following surgery the patient was restarted on FOLFOX with bevacizumab for an additional 2 cycles. Repeat imaging demonstrated residual but stable disease in the right liver, and no other evidence of metastatic disease. Following multidisciplinary tumor board discussion, the patient was then taken for right hepatectomy. Surgical pathology revealed no evidence of viable carcinoma and hepatic parenchyma with histologic features consistent with treatment effect. At this time, 15 months following diagnosis, the patient demonstrated no evidence of disease and CEA was within normal limits at 1.5 ng/mL. Following right hepatectomy, the patient received 2 additional cycles of FOLFOX and then transitioned to surveillance follow-up every 3 months. Nine months following systemic therapy, imaging demonstrated a solitary liver metastasis and there was a slight rise in CEA to 4.0 ng/mL. Following multidisciplinary tumor board discussion, it was determined that SBRT was the best treatment option given the patient’s single site of disease.
The patient received 54 Gy in 3 fractions to the 3.6-cm-x-2.7-cm segment II lesion. Treatment was delivered over 8 days (minimum 40-hour interfraction interval) with volumetric arc therapy (VMAT) using 10 MV flattening filter-free photons. The patient was treated at 80% maximum inhalation breath-hold utilizing Automatic Breathing Control (ABC) (Elekta, Stockholm, Sweden). Daily image guidance was performed with ABC-gated cone-beam CT.
The patient tolerated SBRT well with toxicity isolated to grade 2 diarrhea 2 weeks following SBRT. Follow-up CEA decreased to 1.8 ng/mL 1 month after SBRT and imaging 14 months after SBRT demonstrates a complete response in the segment II lesion, and no other hepatic metastases.
Citation
Campbell SR, Balagamwala EH, Woody NM, Stephans KL. Multimodality management of colorectal liver oligometastases. Appl Radiat Oncol. 2019;(3):9-16.
September 4, 2019