Review ArticleHead & Neck cancer
National Trends in the Use of Stereotactic Radiosurgery for Glioblastoma
Images

Abstract
Background: Glioblastoma (GBM) is a high-grade intracranial malignancy with a propensity to progress. We analyzed the National Cancer Database (NCDB) to examine trends in the use of stereotactic radiosurgery (SRS).
Methods: We queried the NCDB for patients with GBM receiving intracranial radiation. Odds ratios were used to determine SRS predictors. Cox regression was used to determine predictors of overall survival (OS).
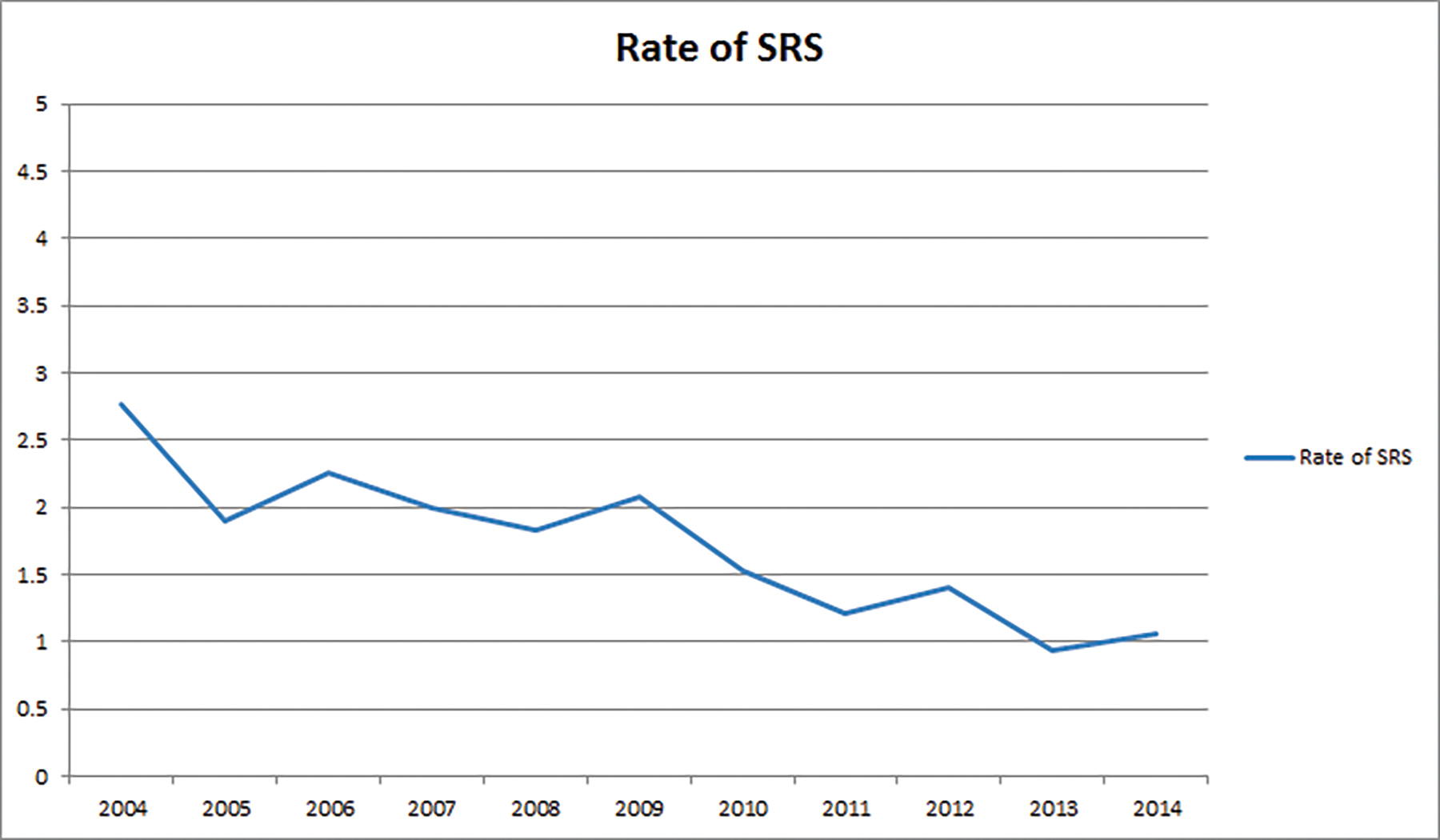
Results: We identified 62681 patients meeting eligibility criteria. Predictors of SRS use were increased age, government insurance, lower comorbidity score, treatment at an academic facility, metropolitan location, and earlier years of treatment. Increased age, lack of chemotherapy, higher comorbidity score, and earlier year of treatment predicted worse OS. SRS utilization decreased from 3% in 2004 to 1% in 2014.
Conclusion: SRS use in the initial management of GBM has steadily decreased.
Glioblastoma (GBM) is the most common primary malignancy of the brain in adults, affecting close to 30 000 patients per year in the United States.1 A locally aggressive tumor, GBM has a high propensity for intracranial progression despite multimodality therapy including maximal safe resection succeeded by adjuvant chemoradiation.2 Given high rates of local failure, attempts have been made to explore the use of escalated doses of radiation, ultimately showing no benefit.3-5 Initially developed by Swedish neurosurgeon Lars Leksell, stereotactic radiosurgery (SRS) represents an advanced method of delivering high-dose-per-fraction radiation treatments in a tightly conformal manner.6 With SRS, conformal dose escalation is achievable and has been investigated in the pre-temozolomide era in RTOG 9305.7 In this trial, patients were treated with an initial SRS boost followed by a course of fractionated external-beam radiation (EBRT). Ultimately, no discernible benefit was observed. It is conceivable that upfront SRS use in GBM management would decline after penetrance of the RTOG 9305 findings; however, data supporting this conclusion are lacking. As such, we examined data from the National Cancer Database (NCDB) to analyze trends and potential predictors for the use of SRS in the treatment of GBM.
Methods
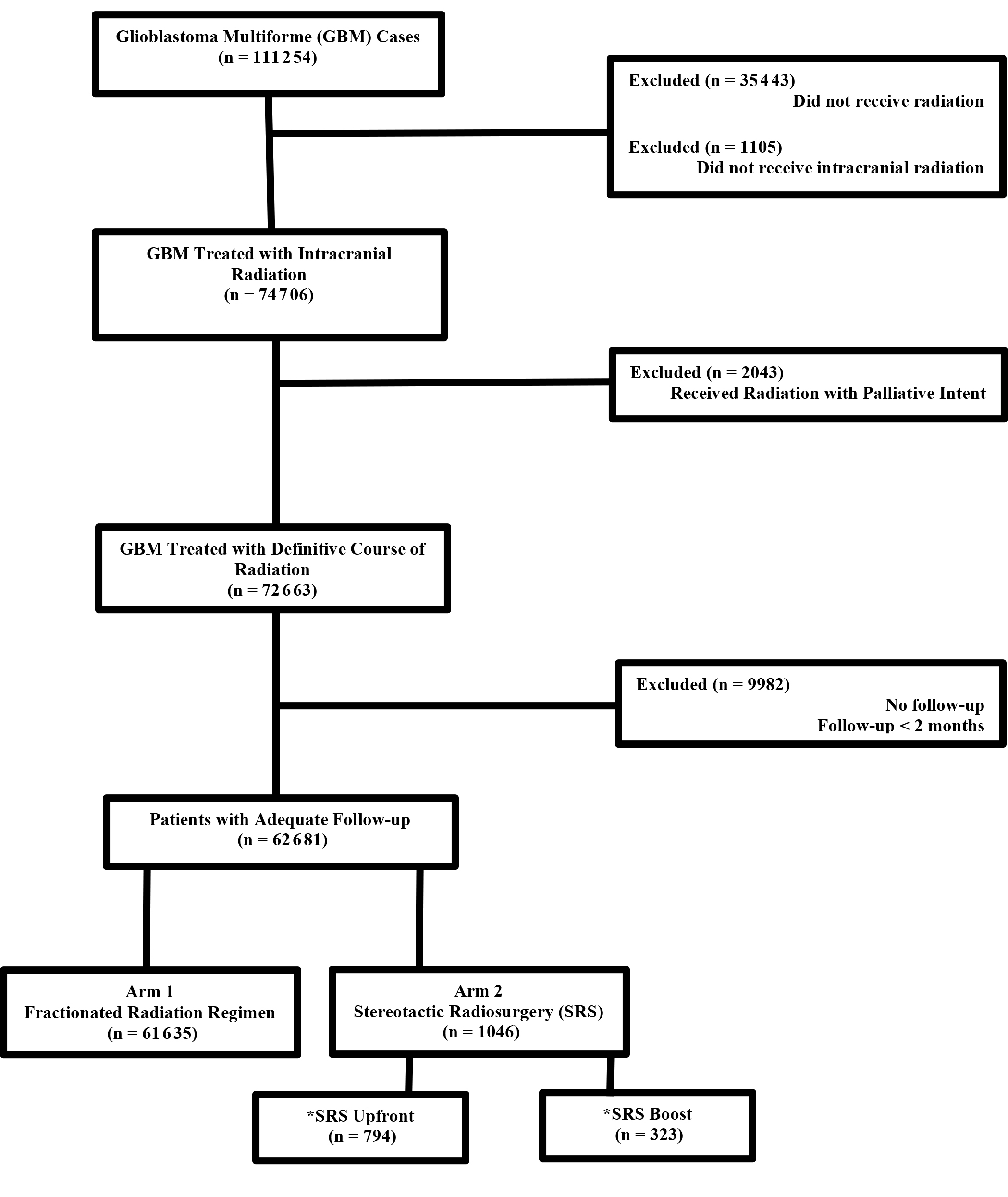
The methods for performing an analysis of the NCDB have been described previously.8,9 We conducted a retrospective review using data from the NCDB, which is de-identified and thus exempt from Institutional Review Board oversight. The NCDB is a tumor registry jointly maintained by the American Cancer Society and the American College of Surgeons for more than 1500 hospitals accredited across the United States by the Commission on Cancer (CoC). It is estimated that this database captures up to 70% of newly diagnosed malignancies each year across the country. We queried the NCDB from 2004-2014 for patients with GBM who had external-beam radiation to the brain delivered with nonpalliative intent (a variable that is recorded within the NCDB). Patients had to have at least 2 months of follow-up. SRS is coded as a specific radiation technique within the NCDB and used to identify those patients. Figure 1 shows a CONSORT (Consolidated Standards of Reporting Trials) diagram outlining the selection criteria and inclusion/exclusion.
Race was broken down into three broad categories: Caucasian, African American, or other. Comorbidity was quantified using the Charlson/Deyo comorbidity index.10 Stage was defined according to the 7th edition of the American Joint Committee on Cancer’s clinical group. Socioeconomic data in the patients’ residence census tract was provided as quartiles of the percentage of persons with less than a high school education and median household income. The facility type was assigned according to the CoC accreditation category. Locations were assigned based on data provided by the US Department of Agriculture Economic Research Service. Insurance status is documented in the NCDB as it appears on the admission page. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the CoC have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Data were analyzed using MedCalc Version 18 (Ostend, Belgium). Summary statistics are presented for discrete variables and c2 tests compared sociodemographic, treatment, and tumor characteristics between the treatment groups. Overall survival was calculated in months from time of diagnosis to date of last contact or death, which is the standard way this data is recorded in the NCDB. Kaplan-Meier curves were used to calculate cumulative probability of survival.11 Log-rank statistics were used to test whether there was a statistically significant difference in the cumulative proportions across groups. A Cox proportional hazards model was used for multivariable survival analysis.12 Due to the large nature of the dataset, factors significant on univariable Cox regression were entered using a stepwise backward elimination process. Adjusted hazard ratios and 95% confidence intervals are reported, using an α level of 0.05 to indicate statistical significance.
Propensity score-adjusted survival analysis was used to account for indication bias due to lack of randomization between patients receiving standard external-beam radiation and SRS.13 Multivariable logistic regression was used to calculate a propensity score indicative of conditional probability of receiving standard radiation or SRS. The propensity model included observable variables associated with treatment selection on multivariable logistic regression. A Cox proportional hazards model was then constructed incorporating the propensity score, but also excluding factors included in the propensity score calculation to avoid overcorrection. The assumption of balance was further validated by stratifying the data into propensity score-based quintiles, and confirming that the difference in propensity score mean per quintile was < 0.10.
Results
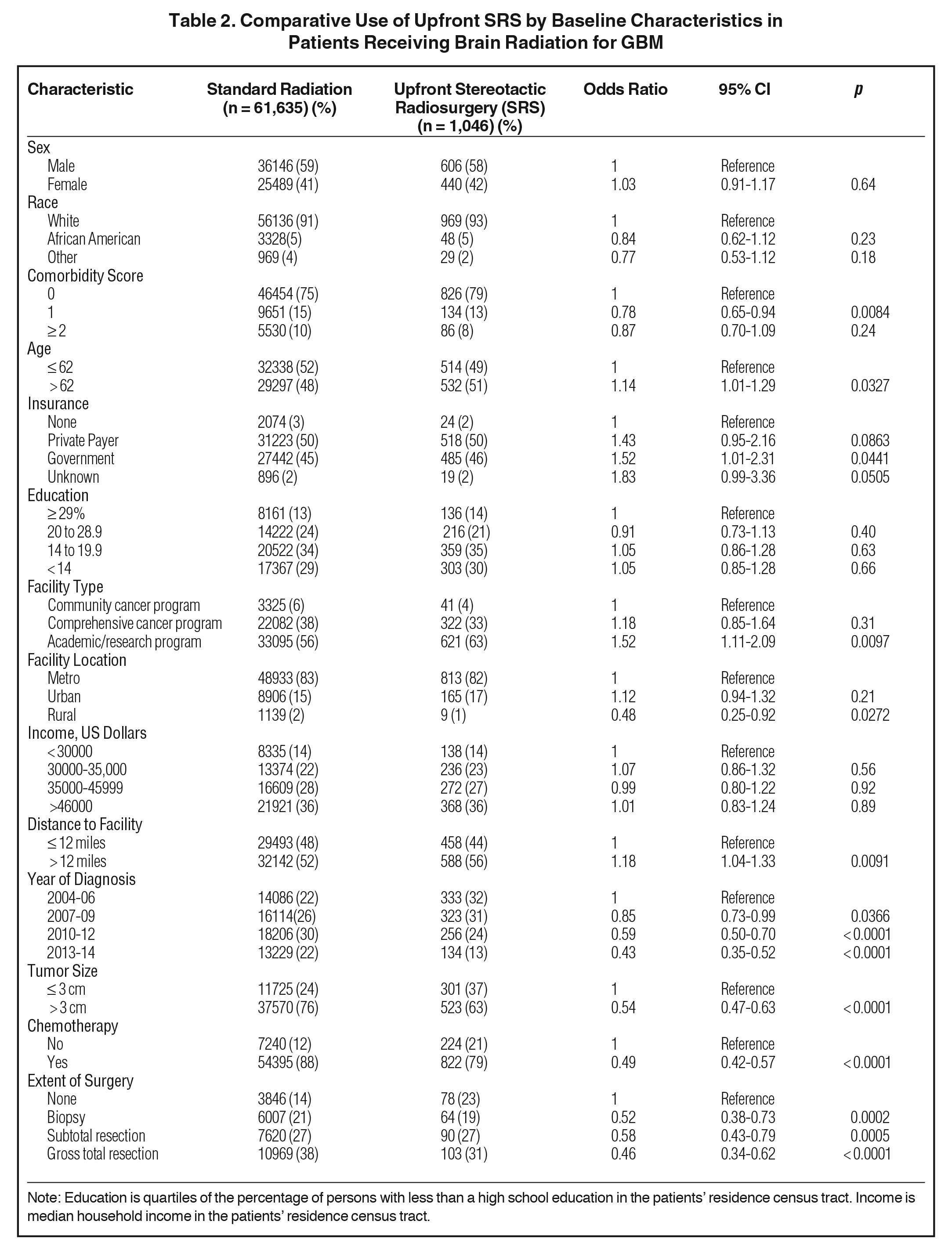
We identified 62681 patients meeting the above eligibility criteria, with 1046 patients receiving SRS as part of initial treatment. Table 1 displays patient characteristics of the population. Of note, Karnofsky Performance Status (KPS) and MGMT (O6-methylguanine-DNA methyltransferase) methylation status were only documented after 2010, with data present in < 10% of cases. As such, these were recorded in Table 1, but not used for statistical analyses. In addition, isocitrate dehydrogenase (IDH) status is not recorded in the NCDB and thus not tabulated. Almost 90% of patients received chemotherapy. SRS use was sparse, and decreased over time, from 3% in 2004 to approximately 1% in 2014 (Figure 2). Predictors of SRS were increased age, government insurance, lower comorbidity score, treatment at an academic facility, metropolitan location, increased distance to facility, smaller tumor size, lack of surgery, no chemotherapy, and more distant year of treatment (Table 2). In addition, multivariable logistic regression identified lack of chemotherapy, increased distance to facility, smaller tumor size, treatment at an academic center, and lack of surgery. The median dose in the SRS group was 40 Gy (interquartile range: 16.2 to 66.2 Gy). The median dose in the standard arm was 60 Gy (interquartile range: 59.4 to 60 Gy). The median time to start of radiation was 32 days (interquartile range: 24 to 42 days) and 28 days (interquartile range: 17 to 40 days) for standard radiation and SRS, respectively. The median time to start of chemotherapy (if given) was 30 days (interquartile range: 20 to 42 days) and 30 days (interquartile range: 19 to 46 days) for standard radiation and SRS, respectively.
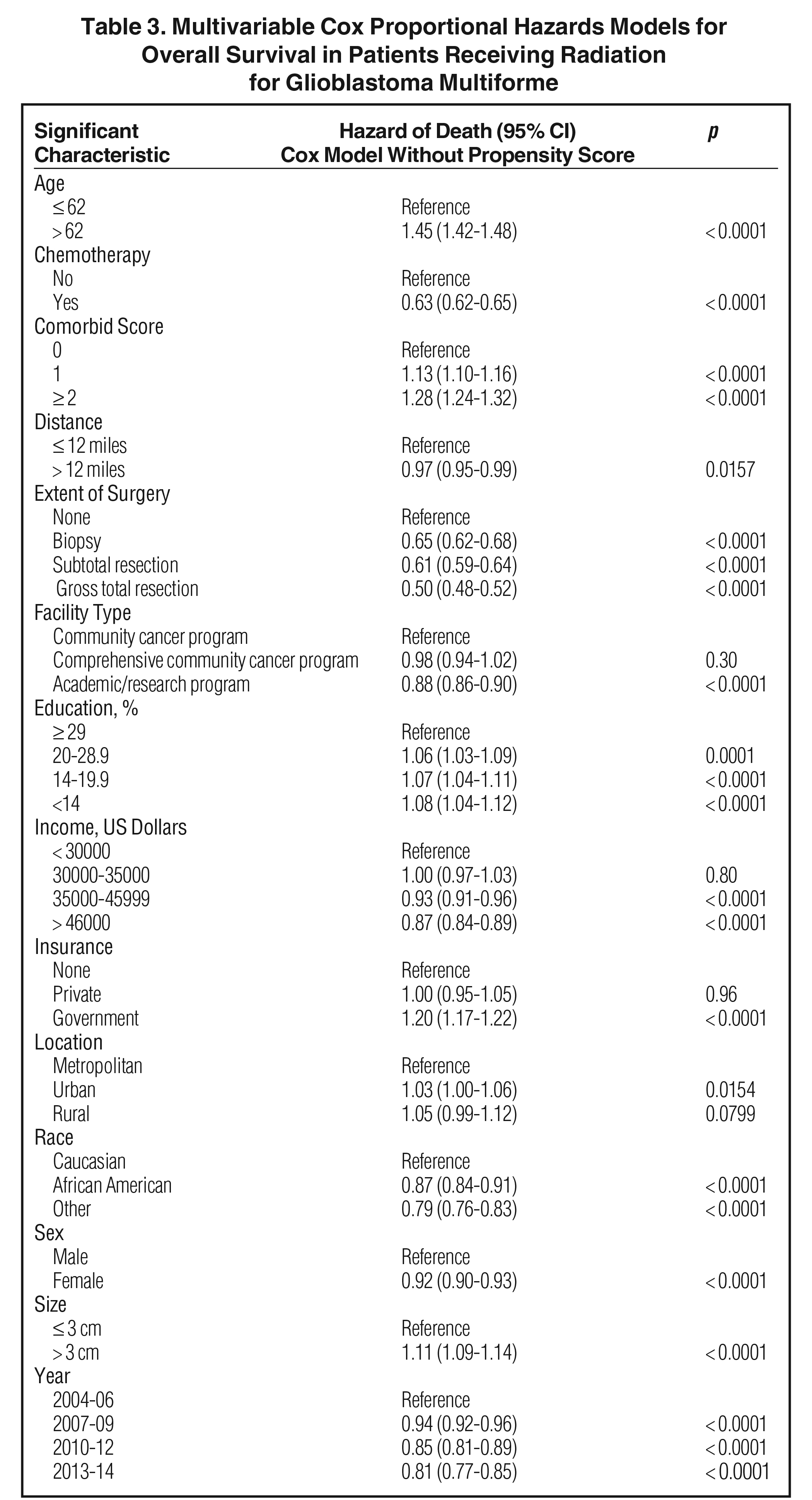
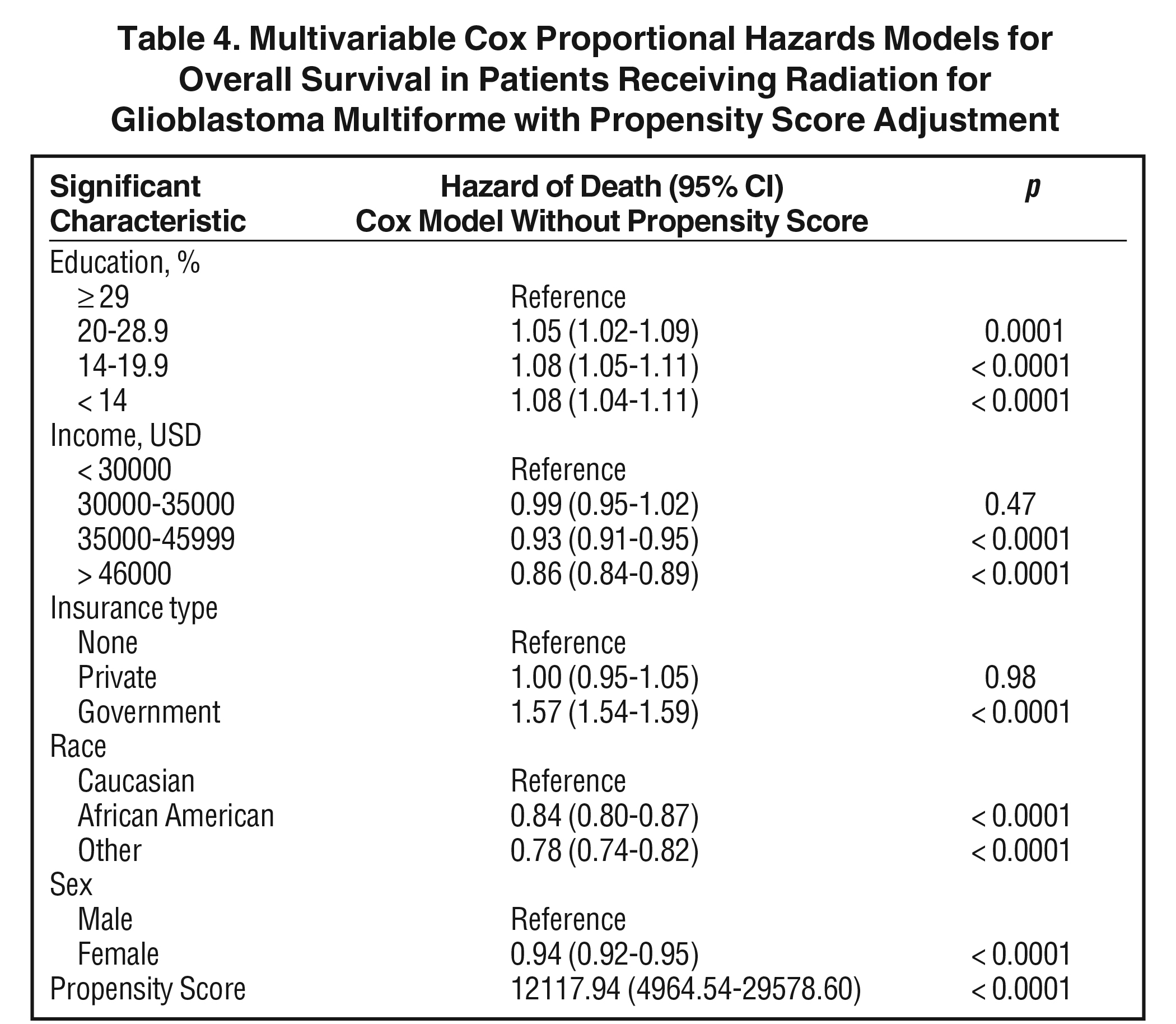
The median follow-up for the entire group was 12.6 months (range: 2 to 155 months). Median follow-up for standard radiation was identical to that of the entire group. Median follow-up in the SRS cohort was 12.6 months as well (range: 2 to 126). Median overall survival was 13 months for all patients, with a 5-year survival of 7%. On univariable analysis, median overall survival was 12.9 months with SRS, compared to 13.1 months with standard fractionated EBRT (p = 0.28) Five-year overall survival was 7% in both groups. On multivariable analysis, increased age, lack of chemotherapy, higher comorbidity score, extent of surgery, treatment at nonacademic facilities, less education, government insurance, urban location, Caucasian race, male gender, larger tumor size, and more distant year of treatment predicted for worse overall survival (Table 3). Use of SRS was not a significant predictor of survival on this multivariable Cox regression. As described in methods, a logistic regression was used to generate a propensity score. The logistical regression model included age, chemotherapy, comorbidity score, distance to facility, surgery, facility type, location, tumor size, and year. Multivariable analysis with propensity score included was then used to determine predictors of outcome (excluding factors used to generate propensity score). On propensity-adjusted multivariable analysis, decreased education, less income, government insurance, Caucasian race, and male gender predicted for worse survival (Table 4).
Discussion
The results of our NCDB analysis confirm a decrease in the use of SRS in the initial management of GBM. In 2004, a limited number of patients (3%) received SRS, with a further decrease to < 1% by 2014. The results of the previously discussed studies support our findings of decreased national use of SRS in the upfront treatment of GBM. In addition, the present analysis did not show any difference in survival between patients treated conventionally and those receiving SRS, which is consistent with previous reports. Regardless, SRS remains an important tool in the retreatment of GBM after local failure as evidenced by multiple contemporary studies.14 -16 Furthermore, based upon the national patterns of SRS use observed in our study, it may be reasonable to consider SRS in elderly patients, patients residing far from treatment facilities, or patients with logistical issues relating to transportation.
Despite advances in imaging, surgery, radiation, and systemic therapy, GBM continues to have disappointing outcomes with 5-year survival in the realm of 10%.1,2,17 The current standard of care for patients with reasonably good performance status is maximal safe resection followed by adjuvant temozolomide-based chemoradiation.18 Despite this aggressive multimodality approach, local failure represents a significant challenge. An investigation by Dobelbower et al assessed survival outcomes and patterns of failure in nearly 100 GBM patients treated with reduced radiation field sizes.4,5 Patients ultimately received a conventional fractionated radiation dose of 60 Gy; however, nearly 90% of patients in this cohort experienced recurrence within the radiation field or marginally. Similarly, a group from Italy reported on outcomes in > 100 patients treated to 60 Gy with concurrent temozolomide.19 Corroborating the findings of Dobelbower et al, progression occurred centrally, in-field, or marginally in approximately 90% of cases.19
Given the exceedingly high rates of local failure, there was considerable interest in the use of higher doses of radiation using EBRT. One of the earliest investigations was that of the RTOG 9803.3 This phase I study utilized conventionally fractionated EBRT (ie, 2 Gy daily fractions) with concurrent chemotherapy in the form of biodegradable carmustine (BCNU).3 Following an initial 46 Gy, patients were dose escalated to 66, 72, 78, or 84 Gy. Median survival was greatest in patients receiving 84 Gy (ie, 14 to 19 months depending on tumor volume) and lowest in the 66 Gy arm. Of note, no dose-limiting toxicities were observed. The authors concluded that dose escalation was feasible and safe; thus, they suggested a potential larger future role with technologic and therapeutic advances. A more recent study from Washington University compared outcomes in patients < 70 years of age who received 60 Gy or > 60 Gy with concurrent temozolomide.20 More than 200 patients were analyzed with the authors identifying age, performance status, and extent of resection as prognosticators of survival. No difference in overall survival was observed at 5 years between the conventional dose and dose-escalated arms; therefore, the authors concluded that dose escalation with temozolomide did not improve outcomes.
Given the highly conformal nature of SRS and its ability to deliver high-dose-per-fraction radiation treatments, it was naturally explored as a potential solution for dose escalation. One of the initial investigations using SRS for dose escalation was RTOG 9305, a randomized multiple institutional study.7 A total of 203 patients were randomized to SRS followed by EBRT to a dose of 60 Gy with concurrent BCNU or conventional treatment with EBRT to a dose of 60 Gy with concurrent BCNU.7 With 5 years of follow-up the median survival in both arms was 13 months, with no difference in failure patterns. A more recent study (RTOG 0023), explored postoperative radiation treatment. Patients were treated with EBRT to a dose of 50 Gy succeeded by SRS delivered once per week at 5 to 7 Gy fractions for a total of 4 weeks.21 Patients also received BCNU for 6 cycles in this study. Seventy-six patients were evaluable and median OS was 12.5 months, thus comparable to historical controls. Of note, both aforementioned studies were in the pre-temozolomide era. More recently, other groups have experimented with hypofractionation with concurrent temozolomide as a means of dose escalation. One study examined outcomes in 24 patients treated with 60 Gy in 10 fractions with temozolomide.22 As expected, most patients progressed (71%) but of those, only 50% were central, in-field, or marginal. The median overall survival of 33 months was slightly improved compared to historical controls.
The present study is not without limitations, many of which are intrinsic to the NCDB, including the retrospective nature of data collection and analysis which inevitably contributes to selection bias. Furthermore, the NCDB lacks important data on outcomes such as toxicity, local failure, type of chemotherapeutic agent(s), and number of treatment cycles completed, all of which play an important role in determining outcome for GBM. Moreover, salvage therapy is also not recorded in the NCDB, which is an important player in long-term outcomes for GBM patients given the high likelihood of failure. Also, KPS and MGMT status were not well recorded in the NCDB (as well as IDH status), and may be areas in which SRS could have potential value (ie, poor performance patients or those who are MGMT unmethylated).
Conclusions
Utilization of SRS in the management of GBM has decreased over time, likely reflecting penetrance of multiple prospective and retrospective studies demonstrating no survival benefit. Concordant with previous findings, overall survival was not improved with SRS in our investigation.
References
- Black PM. Brain tumors. Part 1. N Engl J Med. 1991;324:1471-1476.
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-466.
- Tsien C, Moughan J, Michalski JM, et al. Radiation Therapy Oncology Group T: phase I three-dimensional conformal radiation dose escalation study in newly diagnosed glioblastoma: Radiation Therapy Oncology Group Trial 98-03. Int J Radiat Oncol Biol Phys. 2009;73:699-708.
- Dobelbower MC, Burnett III OL, Nordal RA, et al. Patterns of failure for glioblastoma multiforme following concurrent radiation and temozolomide. J Med Imaging Radiat Oncol. 2011;55:77-81.
- Gebhardt BJ, Dobelbower MC, Ennis WH, et al. Patterns of failure for glioblastoma multiforme following limited-margin radiation and concurrent temozolomide. Radiat Oncol. 2014;9:130.
- Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
- Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853-860.
- Hasan S, Renz P, Turrisi A, Colonias A, Finley G, Wegner RE. Dose escalation and associated predictors of survival with consolidative thoracic radiotherapy in extensive stage small cell lung cancer (SCLC): a National Cancer Database (NCDB) propensity-matched analysis. Lung Cancer. 2018;124:283-290.
- Hasan S, Renz P, Wegner RE, et al. Microsatellite instability (MSI) as an independent predictor of pathologic complete response (PCR) in locally advanced rectal cancer: a National Cancer Database (NCDB) Analysis. Ann Surg. 2018.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-619.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481.
- Cox DR. Regression models and life - tables. J Royal Statist Soc. 1972;34:187-220.
- D’Agostino RB, Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265-2281.
- Gigliotti MJ, Hasan S, Karlovits SM, Ranjan T, Wegner RE. Re-irradiation with stereotactic radiosurgery/radiotherapy for recurrent high-grade gliomas: improved survival in the modern Era. Stereotact Funct Neurosurg. 2018:1-7.
- Hasan S, Chen E, Lanciano R, et al. Salvage fractionated stereotactic radiotherapy with or without chemotherapy and immunotherapy for recurrent glioblastoma multiforme: a single institution experience. Front Oncol. 2015;5:106.
- Torok JA, Wegner RE, Mintz AH, Heron DE, Burton SA. Re-irradiation with radiosurgery for recurrent glioblastoma multiforme. Technol Cancer Res Treat. 2011;10:253-258.
- Hottinger AF, Pacheco P, Stupp R. Tumor treating fields: a novel treatment modality and its use in brain tumors. Neuro Oncol. 2016;18:1338-1349.
- Blumenthal DT, Gorlia T, Gilbert MR, et al. Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: a secondary analysis of EORTC and NRG Oncology/RTOG. Neuro Oncol. 2017;19:1119-1126.
- Minniti G, Amelio D, Amichetti M, et al. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol. 2010;97:377-381.
- Badiyan SN, Markovina S, Simpson JR, et al. Radiation therapy dose escalation for glioblastoma multiforme in the era of temozolomide. Int J Radiat Oncol Biol Phys. 2014;90:877-885.
- Cardinale R, Won M, Choucair A, et al. A phase II trial of accelerated radiotherapy using weekly stereotactic conformal boost for supratentorial glioblastoma multiforme: RTOG 0023. Int J Radiat Oncol Biol Phys. 2006;65:1422-1428.
- Reddy K, Gaspar LE, Kavanagh BD, Chen C. Hypofractionated intensity-modulated radiotherapy with temozolomide chemotherapy may alter the patterns of failure in patients with glioblastoma multiforme. J Med Imaging Radiat Oncol. 2014;58:714-721.
Citation
Wegner RE, Abel S, Hasan S, Horne ZD, Verma V, Ranjan T, Yu A, Xu L, Williamson RW, Karlovits SM. National Trends in the Use of Stereotactic Radiosurgery for Glioblastoma. Appl Rad Oncol. 2020;(1):28-34.
April 4, 2020
