New Embolic Device Blocks Vessels Feeding Malignant Tumors
Fluidx Medical Technology, Inc. released details on clinical use of their GPX Embolic Device for blocking flow to small microvasculature and large tumor feeding vessels.
"The versatility of the GPX product has been demonstrated in a variety of interventional oncology uses," said Andrew Holden, M.D., MBChB, FRANZCR, EBIR, ONZM, Director of Interventional Radiology, Auckland City Hospital, Auckland, New Zealand. "We have seen excellent distal penetration and backfilling of larger vessels in our tumor cases."
The GPX Embolic Device is an innovative embolic designed to combine the benefits of other embolics like coils, particles, and liquids with simplified preparation, delivery, precision, and control leading to durable, long-term occlusions. GPX technology is a low viscosity, aqueous-based solution in a syringe that solidifies into a durable embolic material upon delivery without polymerization or dimethyl-sulfoxide (DMSO) precipitation.
"We saw excellent filling of the distal branches and complete tumor devascularization," said Dr. Martin Krauss, M.D., Head of Interventional Radiology, Christchurch Hospital, Christchurch, New Zealand. "The patient exhibited marked decrease in hematuria (blood in urine) following the procedure. Since we were not worried about catheter entrapment, we could take our time and ensure that we occluded all the feeding vessels of the tumor."
GPX is packaged ready-to-use in a syringe, requires less than 1 minute of tableside preparation by the clinician, and may be delivered through standard catheters or microcatheters.* Clinicians can decide at time of care to use GPX alone or as a complement to coils or other embolic technologies.
"GPX is a highly versatile product that has been shown to successfully occlude small microvasculature as well as the larger tumor feeding vessels." said Libble Ginster, CEO of Fluidx Medical Technology, Salt Lake City, Utah USA. "GPX can be delivered using very small microcatheters, designed to give physicians the flexibility to be highly targeted and thorough in devascularizing tumors."
In a recent case at Christchurch Hospital, the GPX Embolic Device was delivered through long, thin microcatheters, including the 2.0Fr Terumo Progreat Alpha 130cm length microcatheter with an inner diameter of 0.019 inches (0.48mm).
Fluidx Medical Technology is a Salt Lake City, Utah based company focused on developing the GPX Embolic Device and other innovative medical technologies.
The GPX Embolic Device is under development and does not have marketing clearance or approval in any market at this time. For investigational use (in New Zealand) only.
*Fluidx Medical Technology data on file.
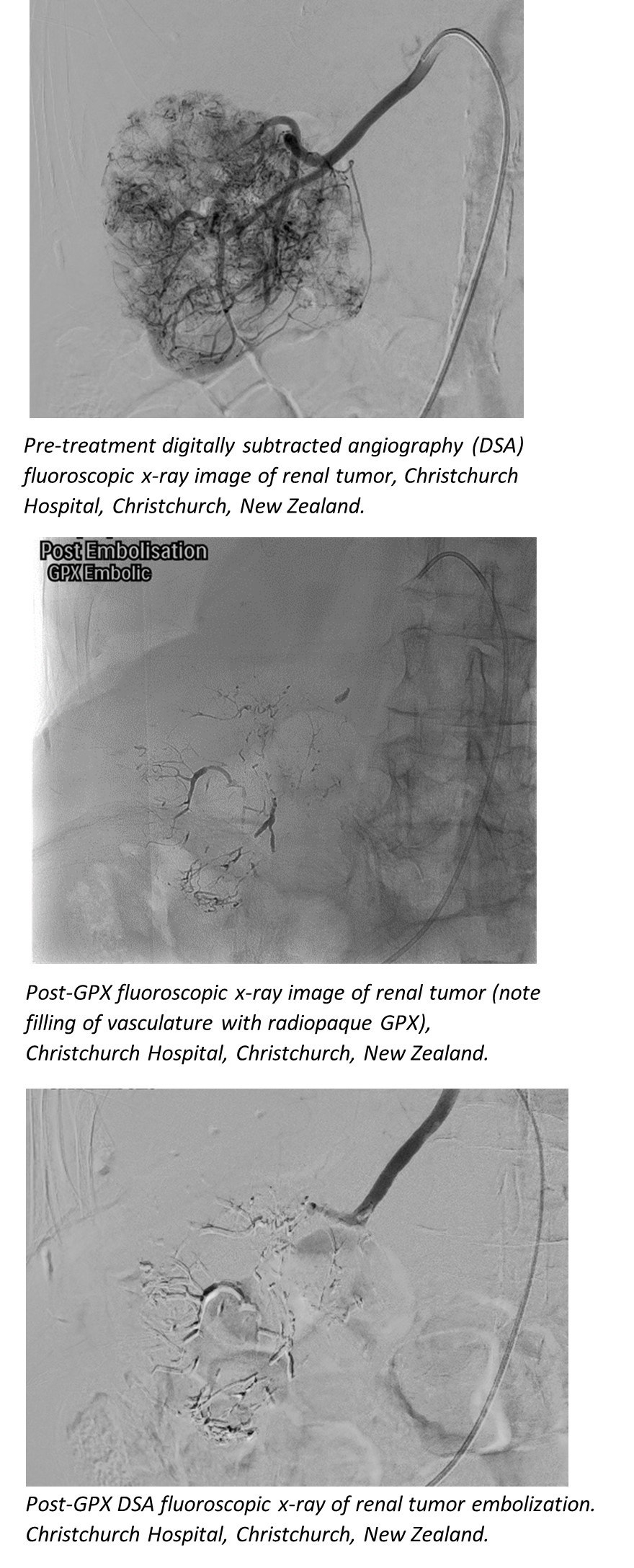
Renal Tumor Embolization Using the GPX Embolic Device, Christchurch Hospital, Christchurch, New Zealand