New PET Imaging Agent Supports Personalized Treatments for Breast Cancer Patients
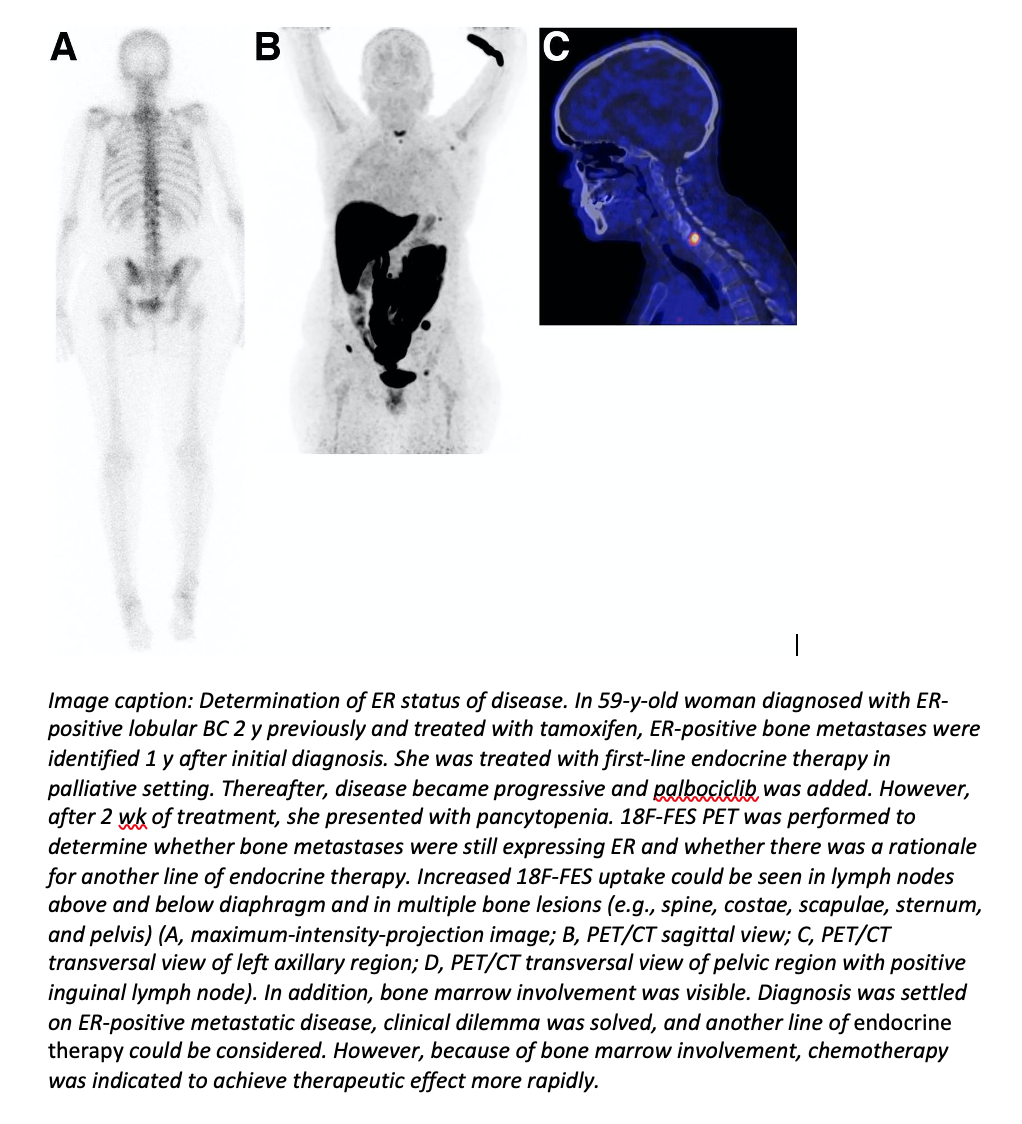 The 18F-FES PET scan, approved by the US Food and Drug Administration in 2020, can help physicians make treatment decisions for breast cancer patients when diagnostic dilemmas arise. The non-invasive, patient-friendly imaging technique was shown to solve 87% of dilemmas, providing physicians with information for personalized treatment decision-making.
The 18F-FES PET scan, approved by the US Food and Drug Administration in 2020, can help physicians make treatment decisions for breast cancer patients when diagnostic dilemmas arise. The non-invasive, patient-friendly imaging technique was shown to solve 87% of dilemmas, providing physicians with information for personalized treatment decision-making.
A key characteristic of breast cancer is the estrogen receptor (ER), which is expressed by 79% of breast tumors. Breast tumors that are ER-positive are likely to respond to hormonal therapy. To assess the presence of the ER, a biopsy of a tumor or metastases is commonly performed. However, clinical dilemmas may arise when a biopsy cannot be performed safely or when discrepancies in ER expression is suspected between tumor lesions.
“18F-FES PET has the potential to visualize the ER expression of all tumors across the body, and could provide helpful information regarding clinical dilemmas in breast cancer patients,” noted Carolina P. Schröder, MD, PhD, medical oncologist at the University Medical Center Groningen in The Netherlands. “In our study, we evaluated whether ER clinical dilemmas could be solved by 18F-FES PET findings.”
The retrospective study reviewed 100 18F-FES PET scans that were performed on 83 patients with three types of clinical dilemmas. These dilemmas included the inability to determine the extent of disease with the standard workup, unclear ER status of the tumor, or the inability to determine which primary tumor caused the metastases.
The clinical dilemma was solved by 18F-FES PET in 87% of scans. In 93% of the solved cases, a treatment decision was based directly on the 18F-FES PET result. The frequency of solved dilemmas was not associated with the type of dilemma. However, the frequency of solved dilemmas was related to ER status, as dilemmas in ER-positive scans were more likely to be solved than in ER-negative scans.
“This is the largest study to show the value of 18F-FES PET in a ‘real daily clinical practice,’” said Schröder. “These findings support the use of 18F-FES PET for optimal patient outcomes and may support broad implementation of 18F-FES PET in clinical practice.”
The study, titled “Value of 18F-FES PET in Solving Clinical Dilemmas in Breast Cancer Patients: A Retrospective Study,” was published in the September issue of The Journal of Nuclear Medicine.