Phase I Trial to Evaluate Safety, Efficacy of Gallium Maltolate for Glioblastoma Multiforme
 A Phase I trial of approximately 20 patients will be conducted at the Medical College of Wisconsin (MCW) to evaluate the safety and efficacy of Gallium Maltolate (GaM) in the treatment of Glioblastoma Multiforme (GBM). The trial, funded by IQ-AI, Ltd., was developed in part based on the results of a preclinical study led by medical oncologist Christopher Chitambar, MD, Emeritus Professor of Medicine and Biophysics, Division of Hematology and Oncology at MCW.
A Phase I trial of approximately 20 patients will be conducted at the Medical College of Wisconsin (MCW) to evaluate the safety and efficacy of Gallium Maltolate (GaM) in the treatment of Glioblastoma Multiforme (GBM). The trial, funded by IQ-AI, Ltd., was developed in part based on the results of a preclinical study led by medical oncologist Christopher Chitambar, MD, Emeritus Professor of Medicine and Biophysics, Division of Hematology and Oncology at MCW.
In the preclinical study, treatment with oral GaM caused a dramatic inhibition of tumor growth that was matched by a significant increase in overall survival. Dr. Christopher Chitambar’s group has shown that GaM kills cancer cells by “hijacking” iron metabolism. Essentially, the cancer cells are tricked into consuming GaM instead of iron, starving the tumor and retarding cancer growth.
GaM is a metal-based compound with anticancer activity that can be administered orally. In prior FDA-approved Phase 1 clinical trials, oral GaM demonstrated low toxicity and was well tolerated. Lawrence R. Bernstein, PhD, is a co-investigator on the trial and will serve as the expert regarding the drug substance and formulations. He will also facilitate sourcing of the clinical-quality GaM. Dr. Chitambar and his colleague, Jennifer Connelly, MD, Associate Professor of Neurology, will be the co-principal investigators of the trial, which marks the first time an oral form of GaM will be used in GBM patients.
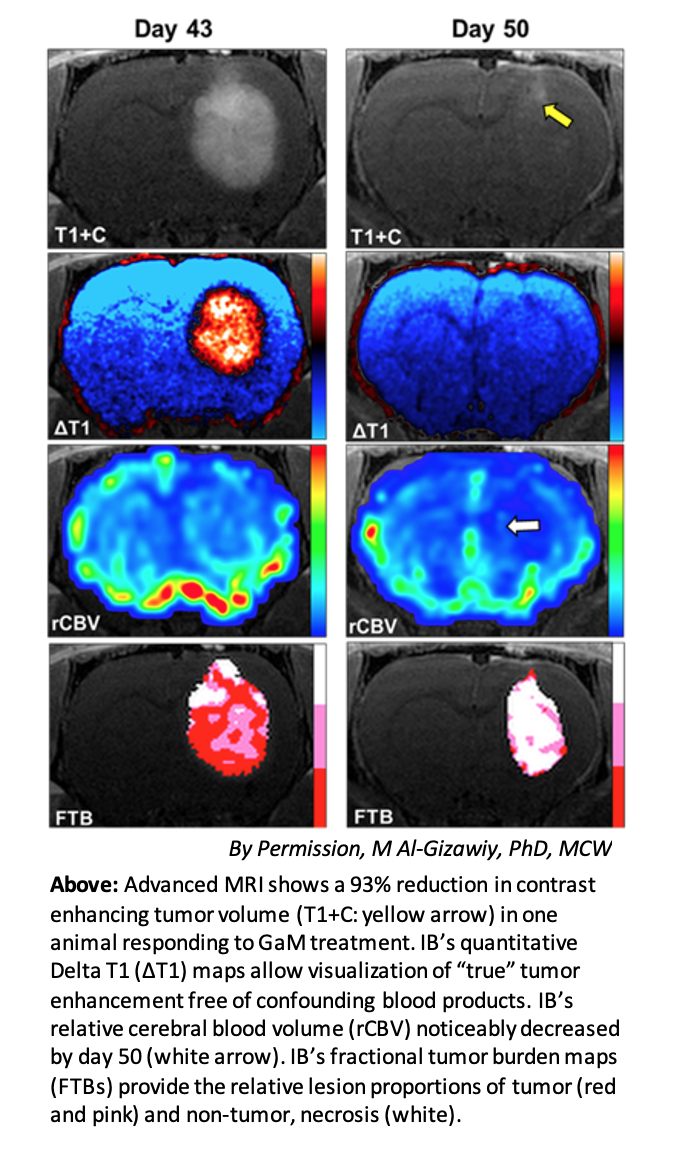
The neuro imaging platform of Imaging Biometrics, LLC (IB), a wholly owned subsidiary of IQAI, will be used to quantitively monitor tumor volumes. The FDA cleared IB Neuro™ and Delta T1™ maps have been used extensively in multi-center clinical trials to assess the efficacy for an array of brain tumor treatments.
If the outcomes look promising, IQAI will enlist a team of regulatory experts in attempt to accelerate the time to market, such as the Orphan Drug program, to benefit patients and their families. “We are taking this one step at a time and need to let the trial progress,” said Michael Schmainda, CEO of IB. “We are working with an excellent team of scientists and clinicians, and everyone is eager to move this study forward.”
GBM is the most common and aggressive primary brain cancer, with limited treatment options and a dismal prognosis. Current treatment involves maximal surgical resection followed by radiation therapy and chemotherapy (bevacizumab and temozolomide). The median survival of patients is only around 14 months. Despite decades of research, only incremental gains have been made to extend or enhance the quality of life.