Phase III Study Reports Telix Kidney Cancer Imaging Agent Detects, Characterizes Renal Masses
 A pivotal Phase III study for Telix Pharmaceuticals Limited’s TLX250-CDx demonstrated the ability to reliably characterize and detect the clear cell phenotype and provide a non-invasive method of diagnosing the presence and spread of clear cell renal cell carcinoma (ccRCC). The results were featured in an oral presentation delivered by Associate Professor Brian Shuch, MD, Director, Kidney Cancer Program, UCLA Institute of Urologic Oncology (Los Angeles, California) and a Principal Investigator in the Phase III ZIRCON study, at the American Society of Clinical Oncology (ASCO) Genitourinary (GU) Cancers Symposium (ASCO GU). It was the first time that detailed analyses of the primary endpoints and key secondary endpoints from the ZIRCON study have been presented to the medical community.
A pivotal Phase III study for Telix Pharmaceuticals Limited’s TLX250-CDx demonstrated the ability to reliably characterize and detect the clear cell phenotype and provide a non-invasive method of diagnosing the presence and spread of clear cell renal cell carcinoma (ccRCC). The results were featured in an oral presentation delivered by Associate Professor Brian Shuch, MD, Director, Kidney Cancer Program, UCLA Institute of Urologic Oncology (Los Angeles, California) and a Principal Investigator in the Phase III ZIRCON study, at the American Society of Clinical Oncology (ASCO) Genitourinary (GU) Cancers Symposium (ASCO GU). It was the first time that detailed analyses of the primary endpoints and key secondary endpoints from the ZIRCON study have been presented to the medical community.
A total of 300 patients were dosed with TLX250-CDx resulting in 284 evaluable patients (those patients with central histology reading and evaluable TLX250-CDx PET scan at central review). Each patient received a single dose of TLX250-CDx and a tumor sample from surgical resection (centrally reviewed) was used as the standard of truth comparator.
The study delivered highly consistent results across three readers of an average 86% sensitivity and 87% specificity, exceeding the pre-determined threshold required to demonstrate the ability of TLX250-CDx to reliably detect the clear cell phenotype and provide an accurate and non-invasive method for identifying the presence and spread of ccRCC. Confidence intervals (CIs) exceeded expectations in all three readers showing high accuracy and consistency of interpretation.
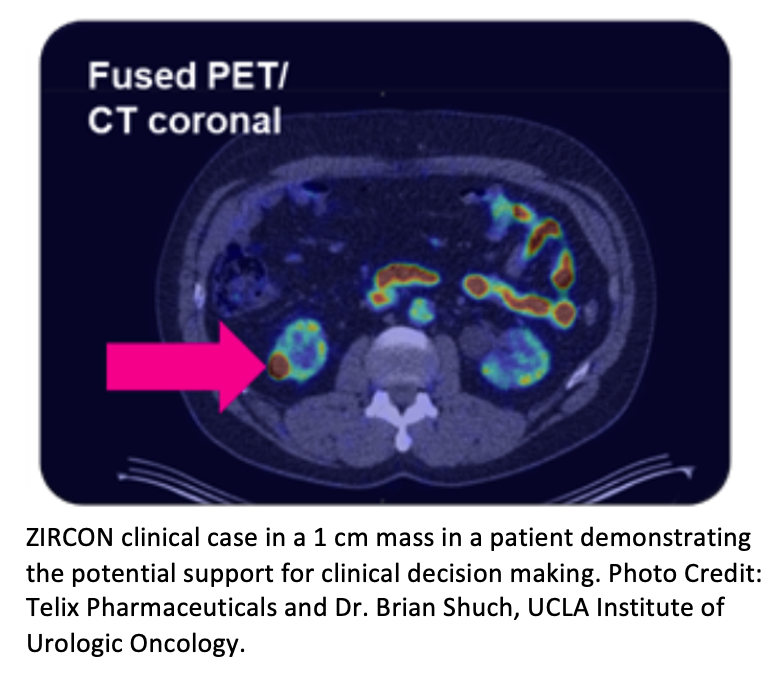
The study also met the key secondary endpoint, achieving 85% sensitivity and 89% specificity in detecting ccRCC in tumors ≤4cm ("T1a" classification), currently a significant clinical challenge in the diagnosis of ccRCC. A clinical case study example was also presented, demonstrating the potential for clinical decision making and accurately identifying clear cell renal cancer even in very small renal masses, smaller than 2cm.
For such cases the high sensitivity and PPV shows that this patient is highly likely to have a ccRCC diagnosis, confirming that they should have this malignant tumor removed. The image could help understand the stage of the disease as well as the location, defining the surgical plan. In such a patient a biopsy would be avoided and they would likely move to surgery with confidence in the diagnosis.
The favorable safety and tolerability profile of TLX250-CDx was also confirmed, with the majority of adverse events (AEs) being post-surgical complications and not study treatment related. No unexpected safety signals were observed and tolerability profile was consistent with experience of girentuximab in previous therapeutic and imaging studies.
Dr. Shuch said, "On behalf of Telix and all of the investigators and clinical sites that contributed towards the successful ZIRCON study, it is a privilege to present at ASCO GU. Since the news of positive top line data in November there has been tremendous interest from peers in the medical community and it's great to be able to dig a little deeper into the clinical impact of these excellent results, including in particular patient sub-sets. The high sensitivity and specificity will allow us to change patient management accurately identifying which patients do or don't have ccRCC."
Dr Colin Hayward, Chief Medical Officer at Telix said, "We are pleased to share these key Phase III ZIRCON study results with the urologic oncology community for the first time at ASCO GU, the leading specialized event for GU cancer care worldwide. The consistency of results and accuracy of the test in both larger and smaller renal masses is especially encouraging. Telix would like to thank Dr Shuch for his personal commitment to this study, as well as all of the patients and clinical teams who participated worldwide."