Portal Vein Stenosis Following Neoadjuvant Therapy With MRgART and Surgery for Pancreatic Cancer: A Case Report
Affiliations
- Department of Internal Medicine, University of Virginia, Charlottesville VA
- Department of Radiation Oncology, Moffitt Cancer Center, Tampa, FL
- Department of Interventional Radiology, Moffitt Cancer Center, Tampa, FL
- Department of Gastrointestinal Oncology, Moffitt Cancer Center, Tampa, FL
- Anderson Publishing, Managing EditorEditorial, 180 Glenside Ave, USA, Scotch Plains
Abstract
Portal vein stenosis (PVS) is a rare but potentially devastating complication arising after definitive treatment of pancreatic cancer. The condition can manifest as symptomatic ascites, abdominal pain, splenomegaly, thrombocytopenia, as well as hemorrhage secondary to gastric or esophageal varices. The etiology is often multifactorial but has been associated with tumor progression, chemotherapy, vascular surgery, and radiation. We present a case in which a man with borderline-resectable pancreatic cancer developed symptomatic ascites secondary to PVS following treatment with neoadjuvant chemotherapy and subsequent 5-fraction MRI-guided adaptive radiation therapy and pancreaticoduodenectomy with vascular reconstruction. Though the incidence of PVS after ablative radiation therapy and surgery for pancreatic cancer appears to be low, it may be under-reported, and patients should be closely monitored in the setting of re-irradiation or planned vascular reconstruction. These findings may help inform future radiation therapy treatment planning guidelines to avoid excessive dose to the portal vein.
Case Summary
A 63-year-old man with a past medical history of alcohol use disorder, recurrent pancreatitis, and colon cancer after sigmoidectomy and no adjuvant therapies presented to the emergency department for pancreatitis with obstructive jaundice. The patient was treated with a biliary stent, and CT showed a 4.0 × 3.8-cm mass located in the pancreatic head with greater than 180° involvement of the superior mesenteric vein (SMV). Endoscopic biopsy confirmed pancreatic adenocarcinoma. The patient was staged as clinical T2N1M0, stage IIB, and the tumor was deemed borderline-resectable after multidisciplinary tumor board review. The patient subsequently underwent 8 cycles of neoadjuvant FOLFIRINOX (fluorouracil, irinotecan, and oxaliplatin), followed by MRI-guided adaptive radiation therapy (MRgART) to 50 Gy in 5 fractions, with the SMV receiving a Dmax of 56.4 Gy ( Figure 1 ). His CA19-9 level decreased from 766 U/mL to 51.8 U/mL, and SMV involvement was less than 180°, making him eligible for pancreatoduodenectomy (PD) with planned en bloc SMV resection with venovenous anastomosis (International Study Group type 3). At 104 days postsurgery and 150 days postradiation, the patient developed symptomatic ascites and CT imaging demonstrated severe stenosis of the SMV at the confluence with the main portal vein (PV). He underwent paracentesis, and 860 mL of ascitic fluid was removed; cytology was negative. Portal venography demonstrated portal vein stenosis (PVS) at the SMV orifice and occlusion of the splenic vein at the confluence with the main PV. Angioplasty of the PVS was performed and resolved the patient’s symptomatic ascites. The patient also experienced variceal bleeding, requiring endoscopic clipping and eventually embolization, and remains in clinical surveillance for pancreatic cancer without evidence of recurrent disease.
MRIdian (ViewRay) treatment plan of simultaneous modulated accelerated radiation therapy dosimetry. Dark blue color wash is the 50 Gy optimization (Opti) structure to gross disease with adaptive dose painting to target and avoid luminal organs at risk with step-and-shoot intensity-modulated radiation therapy. Adjacent portal vein/superior mesenteric vein is contoured in magenta. Other pertinent OARs for adaptive recontouring include stomach (orange), duodenum (pink), small bowel (cyan), and large bowel (green); 110%, 100%, and 60% isodose lines are displayed.
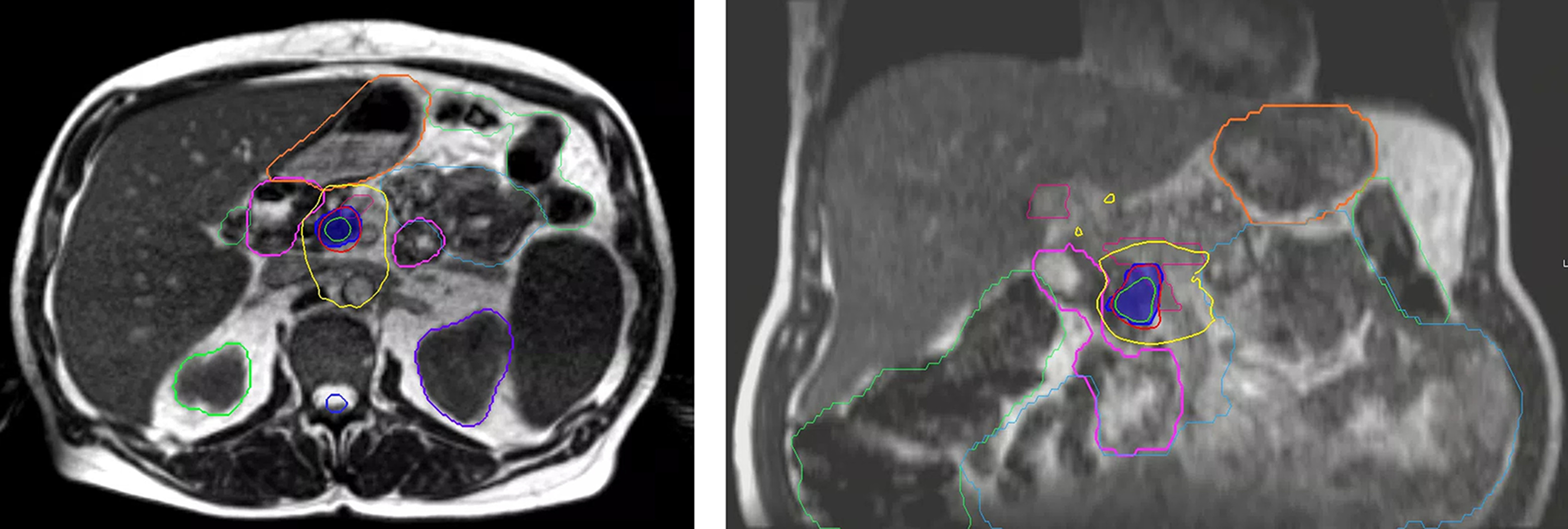
Imaging
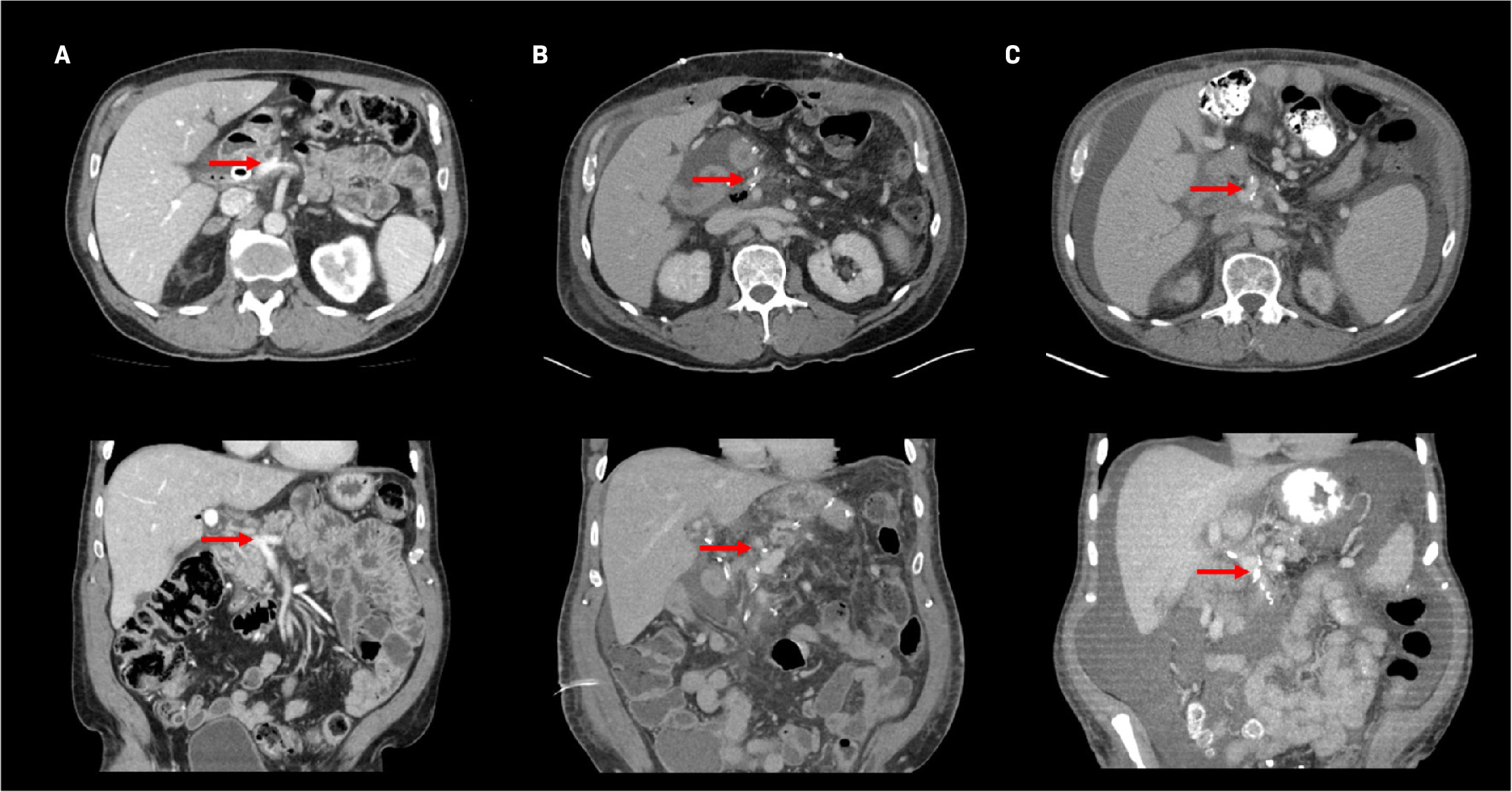
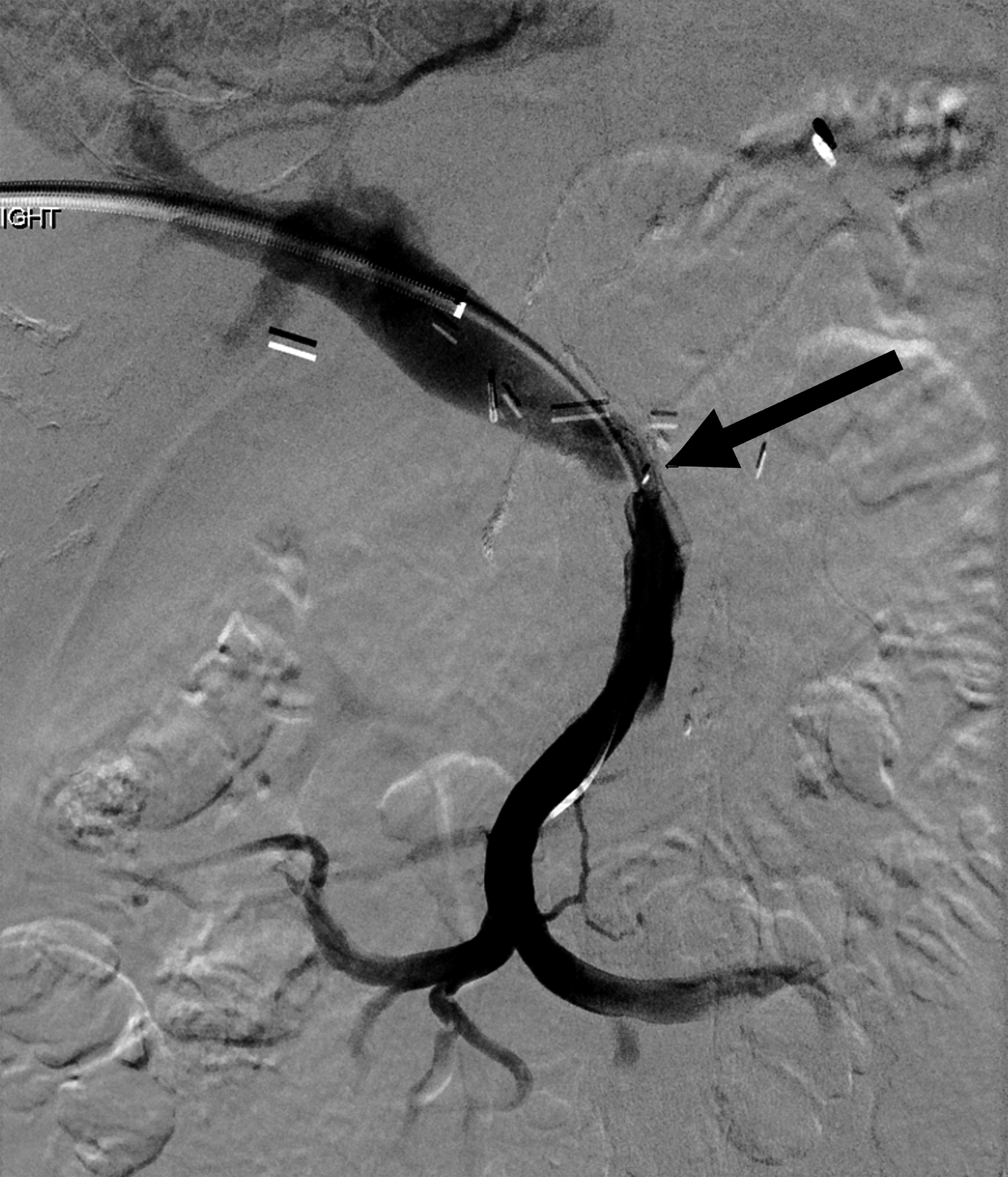
MRI performed as part of the initial diagnostic workup characterized the tumor as a 3.8-cm mass at the pancreatic head, along with a left 1.3-cm periaortic node suspected to be inflammatory. There was vascular encasement of the portosplenic confluence by the tumor as well as greater than 180° contact with the SMV. After neoadjuvant therapy, CT imaging revealed that the pancreatic mass had decreased to 2.6 cm, and there was a decrease in vascular involvement and patent PV ( Figure 2A ). Postsurgical CT showed small-volume ascites, along with narrowing of the PV, SMV, and splenic vein with peripancreatic edema ( Figure 2B ). In surveillance, CT showed increasing ascites and ultrasound showed stenosis at the PV-SMV confluence ( Figure 2C ). The patient underwent paracentesis, and CT demonstrated persistent narrowing of the portal venous confluence. Transhepatic portal venography demonstrated stenosis of the main PV at the SMV orifice and chronic occlusion of the splenic vein at the confluence with the main PV ( Figure 3 ). A timeline of the imaging findings is shown in Table 1 .
Computed tomography contrast-enhanced axial (top row) and coronal (bottom row) images of the portal vein (red arrow) following simultaneous modulated accelerated radiation therapy preoperative ( A ), postoperative ( B ), and at the development of symptomatic ascites (C).
Transhepatic portal venography demonstrated stenosis of the main portal vein (PV) at the superior mesenteric vein orifice (arrow) and chronic occlusion of the splenic vein at the confluence with the main PV.
Imaging Timeline
| DATE | EVENT/IMAGING TYPE | FINDINGS |
|---|---|---|
| September 2022 | Initial presentation | - |
| October 2022 | MRI | 3.8 × 3.2 × 4.0-cm mass in pancreatic head, along with a 1.3-cm left periaortic node. The mass included vascular encasement of the portosplenic confluence with associated narrowing. The mass also abutted both the PV and SMV, with > 180° of contact and contour irregularities associated with both vessels. |
| October 2022 | PET | Negative for regional lymphadenopathy or distant uptake. |
| October 2022 to March 2023 | Chemoradiation | - |
| March 2023 | CT | Pancreatic mass had decreased from 3.8 cm prior to chemotherapy to 2.6 cm. There was a decrease in vascular involvement with respect to the PV and SMV, now with less than 180° of contact in both, although contour distortion was still present with respect to the SMV. |
| April 2023 | Surgery | - |
| April 2023 | CT | Small volume ascites, along with mild narrowing of the PV, moderate narrowing of proximal SMV, and occlusion or near occlusion of splenic vein adjacent to portal vein confluence without evidence of thrombus. |
| April 2023 | US | Patent PV, but the SMV could not be visualized. |
| May 2023 | US | Elevated velocity of the extrahepatic main portal vein, likely due to the superior stenosis at the portal SMV confluence. |
| July 2023 | Paracentesis | - |
| August 2023 | Endoscopic variceal clip | - |
| September 2023 | Transhepatic portal venography | Stenosis of the main portal vein at the SMV orifice. Chronic occlusion of the splenic vein at the confluence with the main portal vein. |
| October 2023 | CT | Significant narrowing of the portal venous confluence. |
Abbreviations: PV, portal vein; SMV, superior mesenteric vein; PET, positron emission tomography; CT, computed tomography; US, ultrasound
Diagnosis
The diagnosis was consistent with symptomatic PVS secondary to occlusion of the splenic vein at the confluence with the main PV, as demonstrated by the imaging that followed the patient’s surgery as well as the evaluation by an interventional radiologist who performed portal venography. The patient only became symptomatic with ascites and esophageal varices after imaging findings began to show PVS. He had no prior history of varices, ascites, or liver failure despite a history of alcohol abuse. Additionally, the imaging taken early in his treatment course showed no evidence of PVS.
Discussion
Here, we describe a case report of symptomatic PVS in a patient with adenocarcinoma of the pancreas treated with chemotherapy, stereotactic body radiation therapy (SBRT) with MRgART, and PD with vascular reconstruction. Following PD, the incidence of iatrogenic PVS was 3.4% to 6.1%, which can be associated with significant morbidity as well as a 3% mortality rate secondary to gastric bleeding.1 - 4 The risk factors for the development of PVS were tumor location in the pancreas, delivery of chemoradiation, and concomitant PV resection.4 The patient, for example, developed both symptomatic ascites and bleeding varices as a result of PVS. Thus, it is important to continue to describe this phenomenon and offer potential etiologies and methods of mitigation.
Pancreatic surgery and porto-mesenteric reconstruction are known risk factors in the development of PVS due to inflammation, narrowing at the anastomotic site, and pancreatic leak.4 - 6 Ten days after PD with vascular reconstruction, 84% of patients have some degree of concentric or eccentric vascular stenosis.6 In patients with pancreatic cancer assessed 5 years after PD, it was found that vascular resection confers 3.28 times the risk of developing PVS — increasing it from 17% to 51%.4
Neoadjuvant chemotherapy may separately partially contribute to PVS. Two common chemotherapy regimens include gemcitabine and oxaliplatin. Gemcitabine monotherapy has been associated with coagulation cascade activation and endothelial damage and has been linked to increased thrombotic events, as well as increased risk when used in multiagent regimens.7, 8 Oxaliplatin has been linked to hepatic sinusoidal obstructive syndrome (SOS) in colorectal cancer due to chronic injury to endothelial cells; however, there is limited literature documenting oxaliplatin-induced hepatic SOS with respect to pancreatic cancer.9 Discussion of this phenomenon may be limited in patients with pancreatic cancer due to more limited survival and, thus, follow-up.
Regarding radiation therapy (RT), radiation-induced vascular inflammation, thrombus, and stenosis are well-described phenomena, and the pathology is generally thought to be limited to small caliber vessels in the myocardium or mandible.10 - 13 Reports on PVS after neoadjuvant chemoradiation are limited7 ; however, a recent prospective study reported the safety of MRgART for patients with locally advanced or borderline resectable pancreatic cancer.14 After neoadjuvant chemotherapy, patients were treated to 50 Gy in 5 fractions and a total of 44 patients (32%) proceeded to surgical resection, with more than half of the patients requiring vascular reconstruction ( n = 23). Two patients experienced grade 5 toxicities of fatal gastrointestinal bleeding that were deemed possibly related to MRgART. Additionally, the 3 postoperative deaths in the study occurred in patients who had vascular reconstruction more than 5 weeks from the completion of radiation. In the study, there were no published PV dose constraints; as a result, we adopted a D0.03 cm3 (maximum dose to 0.03 cm3 ) of 50 Gy for the PV for all patients being treated with 5-fraction RT. We also included vascular injury in the informed consent form when planning RT to an upper abdominal disease site.
Nonetheless, acute-onset PVS may present with rapidly progressive abdominal ascites and endovascular therapies such as angioplasty and stenting can be performed in the setting of iatrogenic or tumor-related PVS. The success rate of stent deployment is approximately 93%, with median patency rates of at least 14 months.3, 15 The primary stent patency in stenting performed for tumor-related PVS was found to be shorter than that for iatrogenic PVS, at 6.5 months compared with 16 months, respectively.15 After stenting, 71% to 93% of patients reported clinical improvements in symptoms, with major complication rates of 0% to 7%.16 - 18 However, rapid symptom recurrence after stent deployment for radiation-induced venous disease within the lower extremities has been described, which suggests radiation changes may make intraluminal stenting less successful compared with stenting of non-irradiated veins.19
We acknowledge several limitations in reviewing and reporting this rare toxicity. The patient had heterogeneous treatments, which make identification of a single inciting factor difficult, and we suspect that the development of PVS is multifactorial. Importantly, the patient’s variceal bleeding occurred prior to ascites, which we suspect was predominantly driven by splenic vein occlusion at the PVS rather than the PVS alone. We also acknowledge that there is likely a much larger group of patients with subclinical PVS without the need for therapeutic paracentesis or endovascular intervention if there is no significant pressure gradient across the vessel. Therefore, the incidence of PVS is likely under-reported in the literature.
Conclusion
Although rarely reported in the literature, PVS is a clinically significant side effect in patients with pancreatic cancer, which may be compounded by multimodality therapy. Although the cause of PVS in this population is not clearly identified and likely multifactorial (eg, extrinsic tumor compression, thrombus, narrowing at the venous anastomotic site, radiation-related vascular changes, and possibly underappreciated vascular and hepatic sinusoidal toxicity from FOLFIRINOX), its incidence and the potential influence of preoperative radiation should not be trivialized. Until clear dosimetric factors mitigating the risk of this phenomenon are more clearly defined, we caution against excessive radiation dose near the PV in operable patients and strongly recommend the adoption of PV dose constraints for patients planned for 5-fraction SBRT.
References
Citation
Adler E, Keit E, Al-Roubaie M, Kis B, Drake JA, Denbo JW, Hodul, PJ, Hoffe SE, Frakes JM, Palm RF. Portal Vein Stenosis Following Neoadjuvant Therapy With MRgART and Surgery for Pancreatic Cancer: A Case Report. Appl Radiat Oncol. 2024;(2):27 - 31.
doi:10.37549/ARO-D-24-00011
June 1, 2024