Protons for prostate cancer: Bragging points, trials and treatment optimization
Images
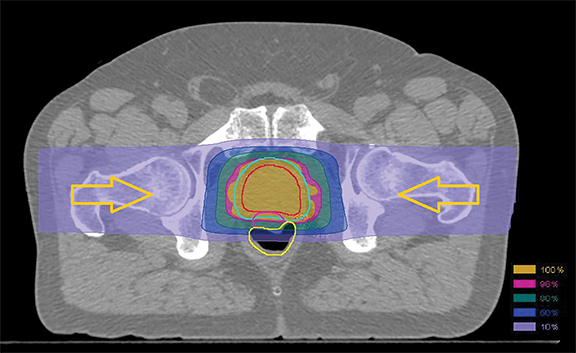

In patients with prostate cancer, advances in photon beam therapy such as 3-dimensional conformal radiation therapy (3D-CRT) and intensity-modulated radiation therapy (IMRT) have helped spare surrounding normal organs and reduce gastrointestinal side effects. Unfortunately, the entrance and exit dose associated with photons results in a significant volume of normal tissue receiving low to moderate radiation doses.1
Enter proton therapy’s “bragging” rights. Under the Bragg peak phenomenon inherent in proton therapy, the radiation beam halts when it hits its target rather than traversing through the patient’s body. In prostate cancer treatment, this spares radiation to outlying areas such as the bladder and rectum, and has helped fuel proton therapy’s growth for this patient population. Nonetheless, some debate—namely regarding expense—surrounds its use.
“What is fueling the controversy is that it costs more,” says Jason A. Efstathiou, MD, DPhil, director of the Genitourinary Division, Department of Radiation Oncology, and clinical co-director of The Claire and John Bertucci Center for Genitourinary Cancers Multidisciplinary Clinic at Massachusetts General Hospital (MGH) in Boston, one of the first hospitals to establish a proton therapy center.
PARTIQol trial
Dr. Efstathiou is also the principal investigator for PARTIQol (Prostate Advanced Radiation Technologies Investigating Quality of Life), a multicenter randomized phase III clinical trial comparing IMRT to proton beam therapy (PBT) to determine which therapy best minimizes treatment side effects and improves quality of life using patient-reported outcomes.2 Trial results should also help reveal whether proton therapy is worth its high price tag, especially given the plethora of management options for prostate cancer, including active surveillance, brachytherapy, prostatectomy and external-beam radiation therapy.
“This is an important question for physicians, patients, policymakers and payers—everyone has a stake in it, and that’s what really led us to open this randomized trial,” says Dr. Efstathiou. “We assume that if we treat to the same biologically equivalent dose, then we can achieve the same cure rates (between PBT and IMRT). So, the brunt of this is quality of life: Can protons deliver fewer bowel effects, less fatigue, less second cancers and improved quality of life?”
The open trial has a goal of 400 patients; 230 were enrolled as of press time, and Dr. Efstathiou expects to finish patient accrual by the end of 2019. In addition to MGH, 11 other U.S. centers are participating: Case Western Reserve University (Cleveland, OH); Mayo Clinic (Rochester, MN); Memorial Sloan Kettering Cancer Center (New York, NY); Northwestern Medicine Chicago Proton Center (Chicago, IL); Princeton ProCure/CentraState Medical Center (Somerset, NJ / Freehold, NJ); Provision Proton Therapy Center (Knoxville, TN); Rutgers Cancer Institute of New Jersey (New Brunswick, NJ); University of Maryland (College Park, MD); University of Pennsylvania (Philadelphia, PA); University of Washington (Seattle, WA); and Washington University (St. Louis, MO). University of Florida will join in late 2017.
The trial allows the use of rectal spacers and moderate hypofractionation, and pencil-beam PBT (Figure 1) is encouraged. “We want the best of the best for each therapy, and to evolve as practice changes,” says Dr. Efstathiou. “We don’t want to be outdated.” He adds that over 10 papers have been published on the physics behind PBT for prostate cancer and biospecimens are also being collected to help identify biomarkers for preferential response.
“Proton therapy has some real potential benefits; [the question is] how to best harness that. At the same time, we need to evaluate it against the best standard of care we have today—IMRT—to see if it’s any better,” says Dr. Efstathiou. “We need to develop the requisite evidence, and simultaneously work to decrease the cost. PBT might be better than IMRT or it may not. But we do need to look at how to make this treatment more cost-effective.”
Lack of insurance coverage for PBT, including participation in PBT trials such as PARTIQol, is another limiting factor. In a 2016 Lancet Oncology commentary, Dr. Efstathiou discussed how the “high frequency of coverage denial severely hinders participant accrual and timely completion of trials, which increases trial costs and skews the study population toward older patients who have Medicare coverage for proton therapy. Thus, despite calls from diverse stakeholders, including patients, physicians, policymakers, and payers, the generation of evidence for proton therapy is being greatly slowed.”3
Patients pay a price as well. If insurance doesn’t cover PBT, many will opt out. “Often, the use of proton beam therapy is dictated by whether a patient’s medical insurance plan covers proton beam therapy or not,” says C. Richard Choo, MD, professor in the Department of Radiation Oncology, Mayo Clinic, Rochester, Minnesota. Launched in 2015, the Mayo Clinic proton beam facility features 4 treatment rooms equipped with pencil-beam scanning and a large proton-beam-generating synchrotron. It features intensity-modulated PBT that uses spot scanning to deposit streams of protons back and forth through a tumor, closely targeting the tumor and sparing healthy tissue.
Contraindications
Contraindications must also be considered, and may rule out PBT as an appropriate treatment option for prostate cancer. Having a bilateral hip prosthesis is a primary contraindication, says Dr. Choo, although patients with a unilateral hip prosthesis can be evaluated on a case-by-case basis. Other contraindications include having an implanted cardiac pacemaker, defibrillator or deep brain stimulator that would be unsafe if turned off; inflammatory bowel disease with active bowel symptoms or inflammation; and prior pelvic radiation therapy causing a field overlap.
“Proton therapy is primarily used when the clinical target volume is limited to the prostate, plus or minus the seminal vessels,” Dr. Choo adds, noting that it is not routine in cases where the clinical volume includes the pelvic lymph nodes and the prostate/seminal vessels.
Addressing uncertainty
In prostate proton therapy, range and position uncertainties, including stopping power, can be accounted for by creating plans using robust optimization. At the Mayo Clinic, Thomas J. Whitaker, PhD, assistant professor, Department of Radiation Oncology, says they review dose volume histogram (DVH) and dose distributions for the nominal plan plus each uncertainty plan. Their standard uncertainties in planning are +/- 3 mm in x, y, and z directions and +/- 3% range uncertainty (variation in stopping power).
Proximity of the rectum to the prostate target volume has always been the primary challenge for prostate radiation therapy by external beams. Hsiao-Ming Lu, PhD, director of clinical physics and associate professor, Department of Radiation Oncology at MGH, has had some success with the use of a rectal spacer, a type of gel injected in the space between the prostate and the rectum that helps separate the areas by 0.5 cm or 1 cm. The gel is absorbed within 6 months and no adverse reactions have been reported.
“That separation helps reduce the dose to the rectum, whether [it originates from a] proton beam or IMRT,” Dr. Lu says. Based on initial use in a limited number of patients, Dr. Lu says that with IMRT a much smaller dose is hitting the rectum, and with PBT there is virtually no dose to the posterior portion of the rectum.
Since the gel features a water-like density, it is implanted under ultrasound-guidance, and must be identified for treatment planning using MR scans, which requires coordination with radiology and MR scheduling. But despite increased workflow requirements, Dr. Lu is fairly confident that MGH will increase use of the rectal spacer.
The spacer may also help with patients who are contraindicated for EBT or PBT due to inflammatory bowel disease or kidney transplants. “With arc-based EBT, there is usually some dose posteriorly,” says Dr. Efstathiou. “If we use laterally based proton beams with the spacer, there may be minimal dose to the rectum, and we may help avoid an inflammatory disease bowel flair.”
At the Mayo Clinic, two anterior oblique fields and two opposed lateral fields are used on patients with rectal spacers, notes Dr. Choo. “When using the 4-field approach, we only treat two fields daily, one lateral and one anterior oblique field,” he says. “Then we alternate the laterality daily.”
An advantage of the anterior oblique fields is reduced radiation to the femoral heads. “In patients with a rectal spacer, anterior oblique fields can be applied more readily because the concern about a higher RBE [relative biological effectiveness] at the end of range landing onto the anterior rectum is lessened, given that a rectal spacer allows a greater separation, ie, distance, between the prostate and the rectum,” he says.
A treatment planning study at MGH investigated anterior-oriented proton beams for prostate cancer and found that it could provide adequate target coverage with dose to the rectum significantly reduced.4 Additionally, a 2015 multi-institution study examined the feasibility of anterior-oriented proton beams for prostate cancer patients with a single or bilateral prosthesis (for whom the standard technique of using only lateral beams was not an option), and found that it provided adequate target coverage and had favorable and acceptable toxicity.5 While the use of spacers would help with anterior oblique beams, it doesn’t necessarily eliminate the problem of end of range uncertainty, says Dr. Lu.
Dr. Efstathiou adds that MGH is exploring the use of anterior and anterior-based oblique proton beams, and says that studies suggest that with a rectal spacer it may be feasible.
RBE throughout the spread out Bragg peak (SOBP) is another area of uncertainty—a “problem that no one really handles well,” says Dr. Whitaker, noting that Mayo uses a Monte Carlo calculation engine that provides a physical dose and a linear energy transfer (LET) distribution (Figure 2). “It is well-established that RBE is proportional to LET, but it is just not well-established what the proportions are,” he says. “We have our own biologic dose calculation created by weighting the physical dose by the LET distribution. This allows us to see potential biologic hot spots and to move away from critical structures.”
RBE uncertainty, especially a higher RBE at the end of the range, is an important factor in determining beam direction and arrangement (lateral beam vs. anterior oblique), adds Dr. Choo, especially in the absence of a rectal spacer.
“Our current practice assumes RBE of 1.1 throughout the entire SOBP,” says Dr. Lu. “There is no clinical data yet, but some clinicians think it could increase to 1.2 near the end of the SOBP, which is substantial. How that will affect the treatment, however, is hard to know at this point.”
Motion matters
While image guidance on traditional EBRT systems has migrated to 3D imaging primarily via cone-beam CT, many PBT systems still rely on 2D orthogonal x-ray imaging. As a result, many sites such as MGH and the Mayo Clinic use implanted fiducial markers as well as rectal water balloons. Preliminary indications are promising in patients with a prostate spacer and in which rectal water balloons are no longer used, but it is not yet clear if the prostate is as stable without the balloons.
“Once we determine our technique with using the spacers, then we’ll look at this closer to see if the spacers help reduce motion,” says Dr. Lu.
The next step in image guidance for proton therapy is cone-beam CT. MGH has purchased a cone-beam x-ray for installation on the PBT system, which may help address beam range uncertainty. “There are efforts now to explore the possibility of using cone-beam CT to evaluate variations of the beam path to control one source of the beam range uncertainty,” says Dr. Lu.
At Mayo, prostate cancer patients have 4 carbon fiducial markers implanted in the prostate prior to treatment. Using on-board 2D orthogonal imaging, the markers are imaged before treatment to confirm setup and account for intra-fraction motion. Dr. Choo says that 3D imaging is also available with the center’s CT on rails for volumetric confirmation of target coverage.
Future study needs
While the PARTIQol trial is expected to make research inroads regarding PBT for prostate cancer, additional investigation is needed. In particular, says Dr. Choo, is the need to compare toxicity and efficacy of PBT vs. photon-based RT, and to evaluate hypofractionation regimens, which can improve PBT cost-effectiveness.
“We also need more radiobiology studies with regard to proton beam—for example, to address issues such as RBE uncertainty,” he adds, and “a need for implantable sensors to accurately and continuously pinpoint the location of tumors in real-time while PBT is being delivered, such as with the Calypso Beacon system (Varian Medical Systems, Palo Alto, California) in a photon therapy setting.”
Ideally, more studies and technological advances will continue to improve PBT, cost-wise and in the clinic, for prostate cancer and additional disease sites.
“Proton beam therapy … has been around a long time [and] is evolving as a technology,” says Dr. Efstathiou. “Yes, it is expensive to build cyclotrons, but we will continue to see smaller and less expensive solutions over time, just as we’ve seen with other technologies like smartphones and computers.”
References
- Wisenbaugh ES, Andrews PE, Ferrigni RG, et al. Proton beam therapy for localized prostate cancer 101: basics, controversies, and facts. Rev Urol. 2014;16(2):67-75. doi: 10.3909/riu0601.
- Proton Therapy vs. IMRT for Low or Intermediate Risk Prostate Cancer (PARTIQoL). https://clinicaltrials.gov/ct2/show/NCT01617161. Accessed Aug. 31, 2017.
- Shah A, Ricci KI, Efstathiou JA. Beyond a moonshot: insurance coverage for proton therapy. Lancet Oncol. 2016;17(5):559-561.
- Tang S, Both S, Bentefour H, et al. Improvement of prostate treatment by anterior proton fields. Int J Radiat Oncol Biol Phys. 2012;83(1): 408-418. PMID: 22133626.
- Cuaron JJ, Harris AA, Chon B, et al. Anterior-oriented proton beams for prostate cancer: a multi-institutional experience. Acta Oncol. 2015;54:868–874. doi: 10.3109/0284186X. 2014.986288.
Citation
MB M. Protons for prostate cancer: Bragging points, trials and treatment optimization. Appl Radiat Oncol. 2017;(3):24-27.
September 21, 2017