Radiation-Induced Optic Neuropathy Following Radiation Therapy for a Recurrent Tuberculum Sellae Meningioma: A Case Report
Affiliations
- Department of Oncology, Oslo University Hospital, Oslo, Norway
- Institute for Clinical Medicine, University of Oslo, Oslo, Norway
- Department of Neurosurgery, Oslo University Hospital, Oslo, Norway
- Department of Radiology, Oslo University Hospital, Oslo, Norway
- Department of Pathology, Oslo University Hospital, Oslo, Norway
- University of Oslo, Oslo, Norway
- Institute for Cancer Genetics and Informatics, Oslo University Hospital, Oslo, Norway
- Anderson Publishing, Managing EditorEditorial, 180 Glenside Ave, USA, Scotch Plains
Abstract
A 62-year-old woman underwent a second surgery for a WHO grade 1 tuberculum sellae meningioma 4 years after her primary resection. The meningioma affected her right optic nerve, and there was a microscopic residual tumor after the second surgery. Due to the history of recurrence, residual tumor, and visual decline, she was offered postoperative radiation therapy of 1.8 Gy in 29 fractions, with a total dose of 52.2 Gy. Maximum doses to the anterior optic pathway structures were 53.7 Gy to the chiasm, 53.3 Gy to the right optic nerve, and 52.3 Gy to the left optic nerve. Following a transient improvement, her vision rapidly worsened 7 to 8 months later, with only finger counting possible in her left eye and a nearly total visual field loss. Visual acuity was reduced to 20/60 in the right eye, the visual field was reduced (especially in the lower 2 quadrants), and radiation-induced optic neuropathy (RION) was suspected. A rare yet disabling condition that may occur following radiation therapy, RION usually presents with painless, rapid visual deterioration in 1 or both eyes. Treatment options are limited, rendering this a devastating radiotherapeutic complication. Systemic steroids were administered to the patient without visual improvement. Bevacizumab was given as a last effort and, after 3 courses, MRI showed some improvement, with regression of presumed inflammatory changes in both optic nerves. However, the patient’s visual function further deteriorated bilaterally. Three additional bevacizumab courses had no effect, neither visually nor radiographically. This case illustrates that despite precautions, including using doses considered relatively safe when planning radiation therapy, RION might develop and may have devastating consequences. Mitigating treatment options are limited.
Case Summary
A formerly healthy 62-year-old woman experienced visual impairment in her right eye. MRI detected a tuberculum sellae mass, which was surgically removed with a presumed gross total resection. Histopathological examination showed a central nervous system (CNS) WHO grade 1 meningioma. Visual function improved following surgery. Four years later, the patient had progressive visual field loss and reduced vision in her right eye. A meningioma recurrence was detected and a re-resection was performed with microscopic residual tumor. Postoperative photon radiation therapy was administered 4 months later. Because of her already affected vision, a cautious fractionation of 1.8 Gy in 29 fractions, with a total dose of 52.2 Gy, was chosen. Unfortunately, her vision rapidly deteriorated 7 to 8 months later bilaterally. Radiation-induced optic neuropathy (RION) was suspected based on the clinical situation and radiological findings. Steroids, followed by bevacizumab, were administered without any clinical improvement, leaving the patient with substantial visual loss ( Figure 1 shows the timeline).
Timeline illustrating the patient’s symptoms and treatment history. GTR, gross total resection; mos, months; RION, radiation-induced optic neuropathy.
Imaging Findings
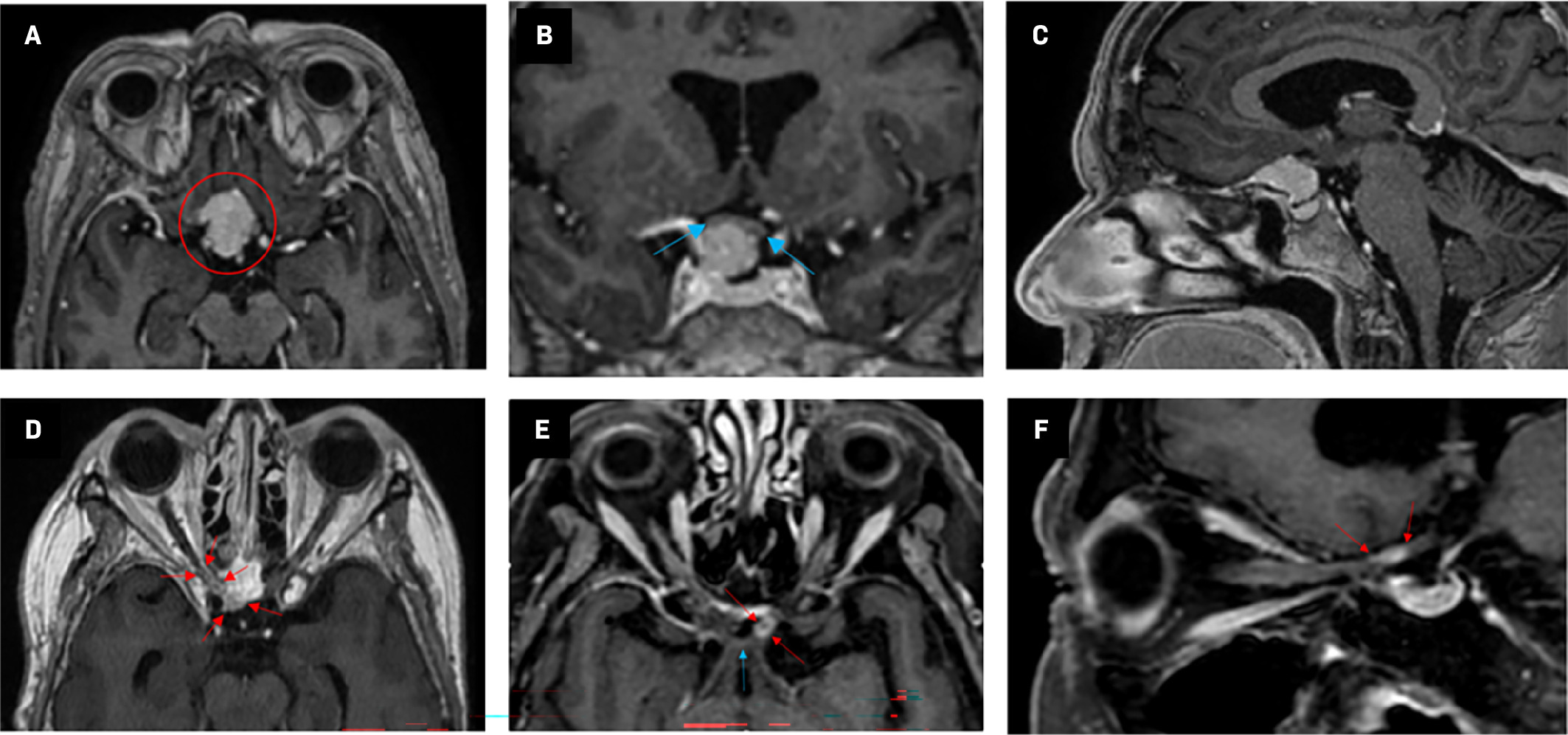
MRI showed a tuberculum sellae mass measuring 18 × 22 × 15 mm compressing the right optic nerve, suspicious of a meningioma ( Figure 2A-C ). The tumor was surgically removed using a pterional approach, with a presumed gross total resection as evaluated by the surgeon and shown in the postoperative MRI. Four years following the first surgery, a tumor recurrence was detected, and MRI suggested that the tumor had grown into the optic canal. A new surgical procedure was performed, and postoperative MRI showed no evident residual tumor. However, the neurosurgeon suspected remaining microscopic tumor tissue in the optic canal. MRI taken as part of radiation therapy planning showed changes compatible with residual tumor alongside the right planum sphenoidale ( Figure 2D ). MRI taken 7 to 8 months following radiation therapy showed contrast enhancement suggestive of inflammatory changes affecting the right optic nerve and surprisingly also showed similar changes in the left optic nerve ( Figure 2E, Figure 2F ).
Axial T1 sequence MRIs with contrast enhancement. (A-C) MRI prior to the first tumor resection. (A) The tumor (red circle) was 18 × 22 × 15 mm in its largest dimension and was at the frontal base of the skull with a slight preponderance to the right. (B) Blue arrows indicate proximity to the chiasm. (D) MRI taken as part of radiation therapy planning. There was a sparse residual tumor (red arrows) alongside the right planum sphenoidale and in the optic canal. (E,F) MRI approximately 9 months following completion of radiation therapy. Left optic nerve contrast enhancement (red arrows) is shown in proximity to the chiasm (blue arrow).
Diagnosis
Prior to the first surgery, the patient experienced progressive visual loss, resulting in a visual acuity of 20/200 in her right eye, and visual field loss in the temporally and lower 2 quadrants. Her vision was described as normal in the left eye prior to surgery. Visual function improved after surgery, and after 3 months visual acuity in the patient’s right eye was assessed to be 20/40. Despite the successful initial surgery, a tumor recurrence was detected 4 years later. At this time point, the patient had reduced vision and a progressive visual field loss in her right eye. After the second surgery, her visual acuity was 20/60 in her right eye and 20/22.5 in the left eye. The right visual field was substantially reduced, especially in the lower 2 quadrants.
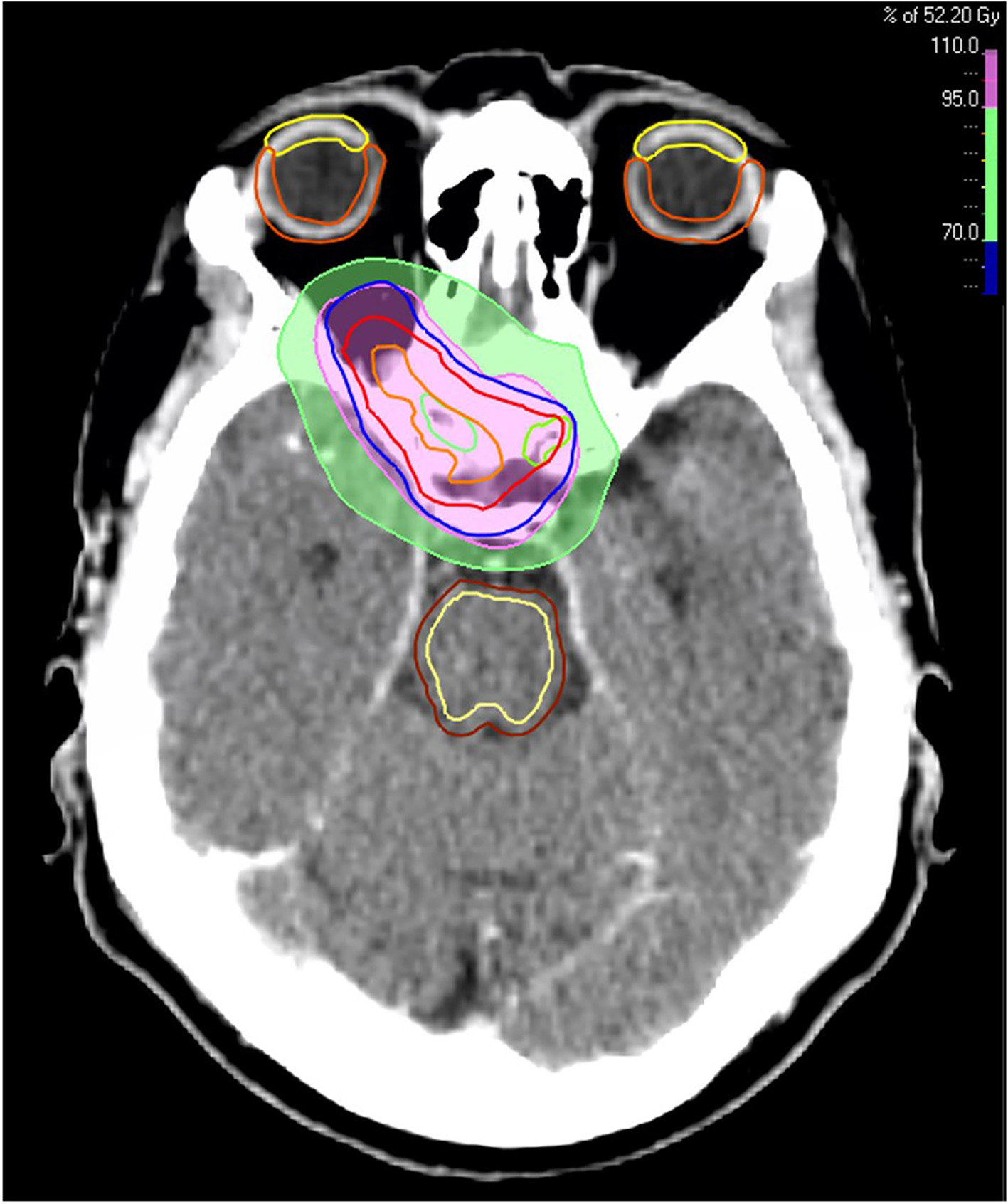
Based on recurrent disease, high probability of microscopic residual tumor remnants, and progressive loss of visual function, the patient was offered postoperative radiation therapy delivered with photons. Radiation therapy was administered 4 months following the last surgical procedure, and a conservative fractionation of 1.8 Gy in 29 fractions, with a total dose of 52.2 Gy, was chosen to keep anterior visual pathway doses below what is considered safe ( Table 1 ) according to the European Particle Therapy Network Consensus. These standards recommend doses below 55 Gy to 0.03 cm3 ( D 0.03cc ) to the chiasm and the optic nerves.1 For the chiasm, the maximum dose ( D max ) was 53.7 Gy and the mean dose ( D mean ) was 51.7 Gy. The right optic nerve had a D max of 53.3 Gy and a D mean of 48.8 Gy, whereas the left optic nerve had a D max of 52.3 Gy and a D mean of 30.5 Gy. Radiation therapy dose distribution is shown in Figure 3 . After radiation therapy, the patient reported minor improvement in visual function. Unfortunately, 7 to 8 months later her visual function again deteriorated over only a few weeks. Both eyes were affected, and deterioration was most pronounced in her left eye, with only finger counting possible and nearly total visual field loss. In the right eye, visual acuity was 20/60, with a substantially reduced visual field, especially in the lower 2 quadrants.
Doses to the Anterior Visual Pathway
| Organ | D max (Gy) | D max EQD2 (α/ β = 2 Gy) (Gy) | D mean (Gy) | D mean EQD2 (α/ β = 2 Gy) (Gy) | Dose constraints (EQD2) as recommended by QUANTEC |
|---|---|---|---|---|---|
| Optic chiasm | 53.7 | 51.0 | 51.7 | 49.1 | D max ≤ 55 Gy |
| Optic nerve, right | 53.3 | 50.6 | 48.8 | 46.3 | D max ≤ 55 Gy |
| Optic nerve, left | 52.3 | 49.7 | 30.5 | 29.0 | D max ≤ 55 Gy |
Key: α/β= alpha/beta; D max = maximum dose; D mean = mean dose; EQD2 = equivalent dose in 2 Gy fractions; QUANTEC = Quantitative Analyses of Normal Tissue Effects in the Clinic.
The patient’s radiation therapy plan, displaying dosage levels of 70% (green area) and 95% (pink area) of 52.2 Gy. Target volumes: orange line (gross tumor volume), red line (clinical target volume), and blue line (planning target volume). Organs at risk: green (optic nerves), yellow (cornea), orange (retina), dark brown (brainstem), and light yellow (brainstem core).
RION is primarily a diagnosis of exclusion, with tumor recurrence being the most important differential diagnosis. Tumor progression often results in a slower course of visual loss than RION.2 The patient’s vision loss was painless and rapid in onset, and radiological findings were bilateral and not consistent with neoplastic progression. Although MRI findings are nonspecific in RION-affected patients, neuro-ophthalmological examinations and clinical course/timing pointed to RION as the most likely cause of the patient’s visual loss. Systemic steroids in high doses were administered for 2 weeks before gradual tapering off, without any improvement in visual function. As a last effort to improve visual function, it was decided to try bevacizumab 7.5 mg/kg every 3 weeks. Treatment was well tolerated, with no reported side effects. Evaluation with MRI after 3 courses showed some radiological improvement, with regression of presumed inflammatory changes affecting the right as well as the left optic nerve. Unfortunately, visual function testing by the ophthalmologist did not improve. Based on MRI findings, it was decided to try another 3 bevacizumab courses, hoping that improvement in vision function would follow with time. The radiological evaluation following the 3 previous bevacizumab courses was stable, whereas the patient’s visual function continued to decline and bevacizumab treatment was discontinued.
Less than 1 year after radiation therapy, the patient’s vision was reduced to finger counting. She had total visual field loss in her left eye and substantial visual field loss in her right eye, with only visual field remnants temporally. Examination revealed large bilateral optic disc retinal nerve fiber layer deficits. Unfortunately, no other treatment options were available and further efforts were focused on trying to improve quality of life with available visual aids.
Discussion
RION is a devastating, albeit rare, complication following radiation therapy and may leave patients with considerable vision loss. It usually presents with subacute, profound, painless, and progressive visual loss. Symptoms typically appear between 10 and 20 months following radiation therapy, with previous data supporting a range from 3 months to 9 years from radiation therapy.2 - 4 Data suggest that one-third of patients develop bilateral RION either simultaneously or sequentially.4 Here, the patient received postoperative radiation therapy based on recurrence and microscopic residual tumor after resection. Although conservative dosing was used, RION evolved bilaterally.
The exact pathogenetic mechanism of RION is unknown. Possible contributing factors are vascular endothelial damage as a result of free radicals from irradiation,5 neuroglial cell progenitors contributing to cellular damage, demyelination, and neuronal degeneration,2, 6 and obstruction of the arteries supplying the optic nerves and chiasm, resulting in optic atrophy.4 Suggested risk factors for developing RION, based primarily on retrospective studies, include high cumulative radiation therapy doses, age (> 60), gender (female), vascular comorbidities, diabetes mellitus, smoking, chemotherapy, previous radiation therapy, pre-existing compression of optic nerves or chiasm by tumor, as well as repeated surgeries.2, 4, 7 For RION to develop following fractionated radiation therapy, cumulative doses normally have to exceed 50 Gy in EQD2 (equivalent dose in 2 Gy fractions). Increased dose per fraction seems to be more important than cumulative doses, and latency may also be shorter with higher fractionation doses.2, 8 Guidelines from the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) recommend a maximum dose ( D max ) ≤ 55 Gy to the anterior optic apparatus, in fraction doses of ≤ 1.9 Gy. According to QUANTEC, RION was unusual for D max < 55 Gy, with an increased risk (absolute risk 3%-7%) for D max 55-60 Gy.3 Parsons et al found that among optic nerves that received doses of ≥ 60 Gy, the 15-year actuarial risk of RION was 11% when the fraction dose was ≤ 1.9 Gy compared with 47% when the fraction dose was ≥ 1.9 Gy.9 Nonetheless, because of uncertainties in calculating doses to anterior visual pathways and individual patient factors, RION should always be considered in patients who develop visual impairment following visual pathway radiation therapy, even if the doses delivered were considered safe.2 Here, the patient is an example of the latter, and she also had some potential risk factors, including female gender, age above 60 years, and vision disturbances prior to radiation probably related to neoplastic compression of the anterior optic apparatus. She did not have hypertension, diabetes, or hyperlipidemia, never received chemotherapy, and was a nonsmoker.
Different treatment strategies for RION such as systemic corticosteroids, hyperbaric oxygen, and anticoagulation have shown little effect.2, 4, 10 Bevacizumab is a recombinant humanized monoclonal antibody targeting vascular endothelial growth factor and has resulted in improvement in some patients with RION, although there is little evidence supporting its effectiveness.8, 11 Interestingly, here the patient had a radiological response after 3 courses of bevacizumab treatment, although her vision continued to deteriorate, leaving her with substantial visual loss. In hindsight, one may argue that the patient would have been much better off with a follow-up MRI and ophthalmologist examinations instead of radiation therapy. On the other hand, RION is an extremely rare complication after radiation therapy delivered with the fractionated regimen chosen for this particular patient. Importantly, for many patients, radiation therapy may prevent further vision deterioration caused by growing meningioma in the optic pathways.12
Conclusion
Radiation-induced optic neuropathy is a rare yet devastating condition following radiation therapy, leaving some patients with substantial visual loss, sometimes bilaterally. Awareness of recommended dose limits to anterior visual pathways and other potential risk factors is important to consider when planning radiation therapy. Even if the doses to optic nerves and the chiasm are below what is considered safe, RION may develop, and it is important to be aware of this during follow-up of patients treated with radiation therapy. Unfortunately, limited treatment options to mitigate RION are available. Further endeavors are needed to identify the risk factors for RION and improve treatment options.
References
Citation
Heggebø LC, Blakstad H, Sprauten M, Magelssen H, Ramm-Pettersen J, Knutstad K, Saxhaug C, Andersgaard A, Leske H, Brandal P. Radiation-Induced Optic Neuropathy Following Radiation Therapy for a Recurrent Tuberculum Sellae Meningioma: A Case Report. Appl Radiat Oncol. 2024;(2):22 - 26.
doi:10.37549/ARO-D-24-00010
June 1, 2024