Case ReportGastrointestinal Cancer
Radiation therapy planning for gastro-esophageal junctional carcinoma in a paraesophageal hiatal hernia
Images
CASE SUMMARY
Although radiation therapy planning, including 4-dimensional (4-D) planning for gastro-esophageal cancer has become standardized, a similar standard has not been well defined for stomach and Siewert type II and III cancers. Rarely, this type of tumor is found in a fixed type II paraesophageal hiatal hernia with the stomach lying in the chest, which makes the planning parameters challenging.1 Distorted anatomy of the stomach, displacement of the heart and lung, and temporal aliasing of the tumor caused by respiratory motion compound the uncertainties of target volume shape and position. Most of such reported cases have been treated with surgery.2-4 Here we report radiation therapy planning for the Siewert type II cancer with the stomach lying fixed in the chest.
An 82-year-old woman with World Health Organization (WHO) performance status 1 presented with melena and anemia. A computed tomography (CT) scan showed a large hiatal hernia with the gastroesophageal junction (GEJ) and a large portion of the stomach displaced into the thorax. Endoscopic biopsy and ultrasound showed adenocarcinoma starting at the GEJ and extending to the proximal stomach with 8 cm in length (30 to 38 cm) and 2.3 cm in thickness invading the muscle without any lymphadenopathy. Positron emission tomography (PET) confirmed this tumor with SUVmax 40 (T3N0M0).
Surgery was discounted due to a high risk of postoperative mortality. Cognizant of her risk of significant side effects, we provided a moderate dose of radiation therapy with a reduced dose of oral capecitabine to control bleeding from the tumor and reduce the risk of dysphagia. The patient received 45 Gy in 25 fractions over 5 weeks with concomitant capecitabine.
RADIATION THERAPY PLANNING
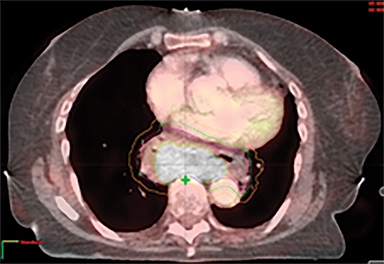
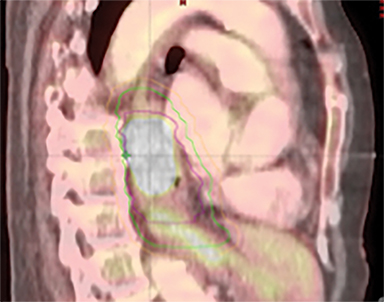
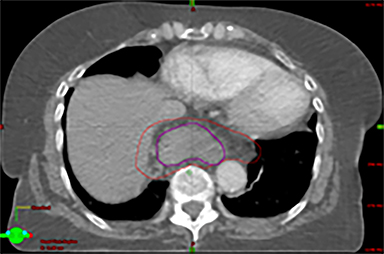
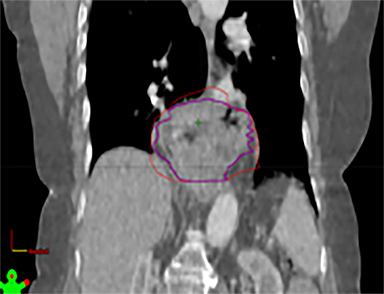
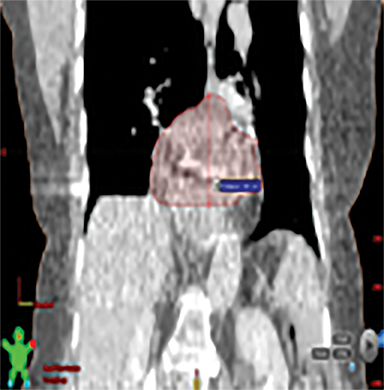
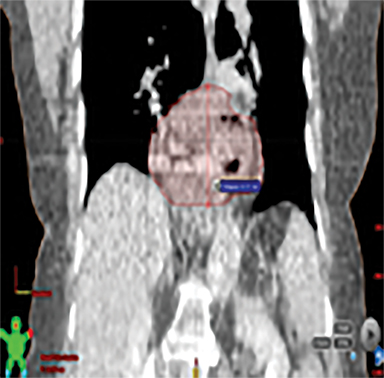
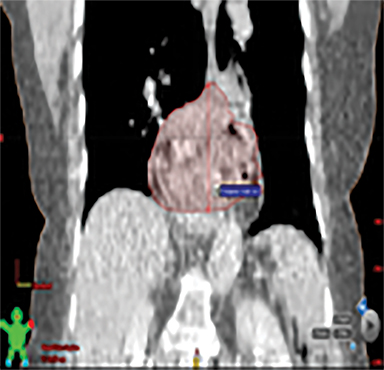
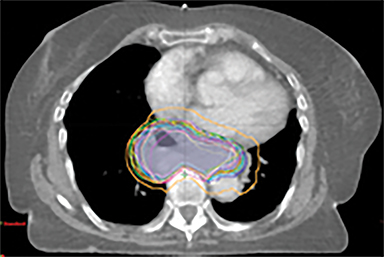
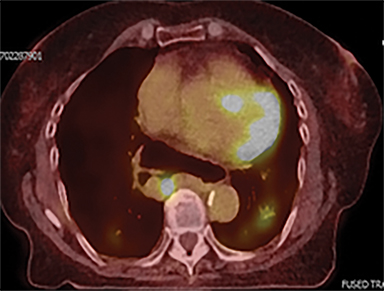
The patient underwent a free-breathing 3-dimensional CT (3DCT) with contrast, and a free-breathing 4DCT scan (binned into 10 phases). The 3DCT, 4DCT, PET 18-fluorodeoxyglucose scan images were co-registered for the target delineation (Figure 1).
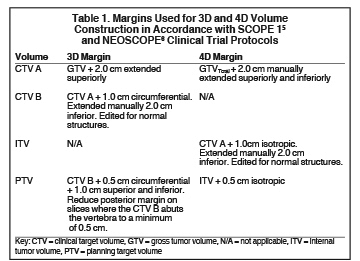
For clinical planning, gross tumor volume (GTV) was delineated on 3 of the 10 phases (max_inhale, max_exhale, and midphase) of the 4DCT scan using PET-CT and endoscopic ultrasound (EUS) information. The GTV from all 3 phases was then combined onto the 3D contrast scan (GTVTotal) and the clinical target volume (CTV) A, CTV B and PTV were produced by applying 4DCT margins as defined in Table 1. Both GTVTotal and CTV A volumes were expanded to account for any additional motion from all other 4DCT phases. The 4DCT scan showed an internal motion of 0.3 cm, 0.5 cm and 0.9 cm in lateral, anterior-posterior (AP) and superior-inferior (SI) directions, respectively.
A RapidArc (volumetric-modulated arc therapy [VMAT]) plan was produced within the Eclipse planning system (V11, Varian, Palo Alto, California) aiming 95% of the prescribed dose to cover 99% of the PTV, keeping organs at risk (OARs) below the constraints. One full arc of a 6-MV beam was used and doses were calculated using the Varian AcurosXB algorithm.
RESULTS AND DISCUSSION
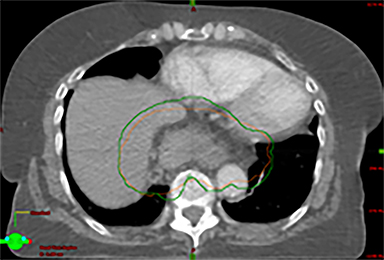
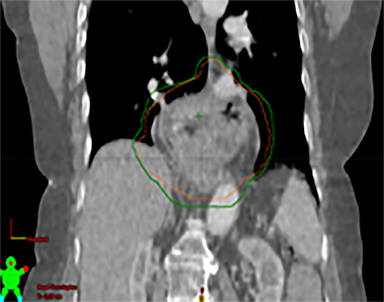
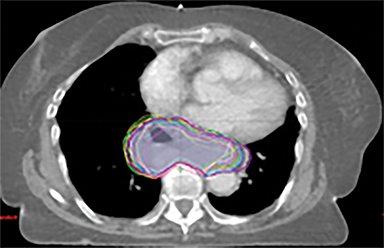
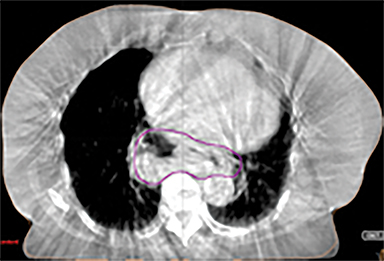
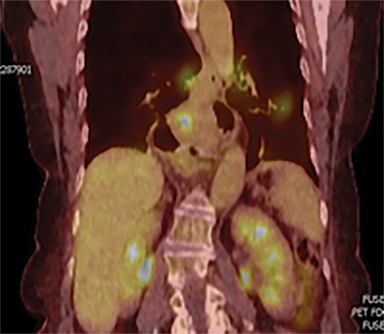
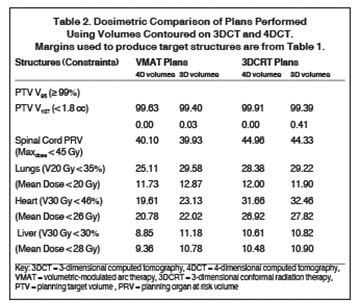
In addition to the clinical plan, the effect of internal target motion on treatment volume and, hence, on OAR doses was assessed by contouring GTV on 4DCT and 3DCT scans separately (Figure 2). Then, 3 additional treatment plans (3D-conformal [3DCRT] and VMAT) were produced with volumes generated using 3D and 4D margins from Table 1, and DVH parameters for all plans were compared (Table 2).
VMAT plans produced on 3D and 4D volumes showed insignificant differences in the PTV coverage; however, a systematic increase in OAR doses was seen for the 3D volume plan. A similar trend was seen for 3DCRT plans; however; mean heart dose exceeded the tolerance dose of 26 Gy in both (3DCRT) plans (Table 2).
Daily free-breathing cone-beam CT (CBCT) was performed prior to each fraction (Figure 2J). Images were matched using bony anatomy and evaluated if the GTV defined at planning was within the PTV. All setup errors were corrected prior to treatment, and the patient completed radiation therapy without any treatment interruptions. The average (max) setup error recorded from the pretreatment CBCT matching for all fractions was 0.1 cm (± 0.7) in the lateral, 0.2 cm ( ± 0.6) in the anterior-posterior (AP), and -0.5 cm (± 0.7) in the SI direction.
Furthermore, interfractional tumor motion was calculated by contouring the GTV volume on all 25 CBCTs (Figure 2). Online registration (ie, the one used for online matching and treatment delivery) was used to transfer the volume on the planning CT. Mean (max) tumor motion was 0.59 (0.86) cm, 0.29 (0.56) cm and 0.45 (0.53) cm in the lateral, AP, and SI direction, respectively. Maximum tumor motion is greater than the margin applied to PTV (0.5 cm isotropic) illustrating the importance of daily CBCT in patients with this condition. Our case also illustrates the bigger lateral rather than SI organ motion, which is observed in the GEJ tumor7,8 in the normally lying infra-diaphragmatic stomach.
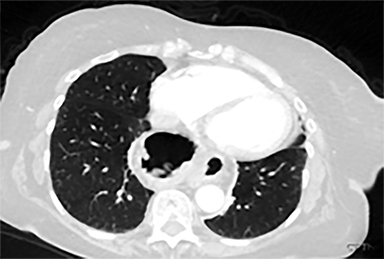
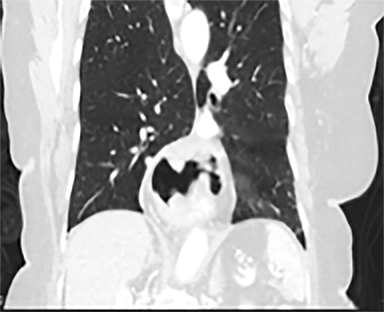
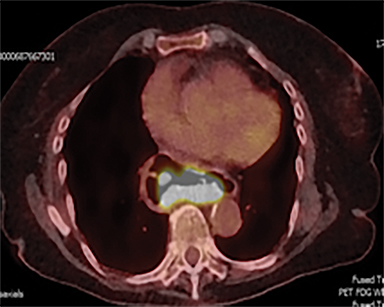
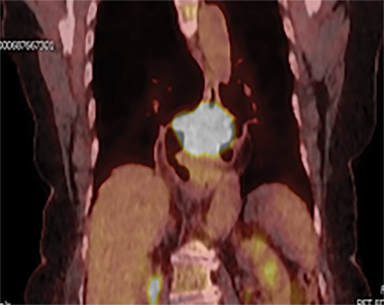
Acute toxicities were grade 1 odynophagia, mild nausea, and grade 1 fatigue. Eight weeks following radiation therapy, the PET scan showed a significant reduction in the volume of hypermetabolic gastric tumor with residual tumor of 1.0 cm and SUVmax of 7 without metastases (Figure 3). The patient’s hemoglobin improved. The patient died due to liver metastases after 7 months following the treatment. During these 7 months, the patient did not require transfusion, remained free from dysphagia and malena, and maintained performance status 1 until 2 weeks before death.
Through a PubMed search, we did not find any 4D radiation therapy treatment planning parameters in a patient with a junctional and upper stomach tumor associated with a large part of the stomach lying into the intrathoracic cavity.
Thoracic and abdominal tumors move with breathing, necessitating that the treatment plan account for motion during treatment planning and delivery.7-9 Organ motion could be high for organs below the diaphragm. The stomach motion was observed mostly in the anterior, superior and left (up to 1.75 cm), toward the right and posterior (0.88 cm), and least inferiorly (0.5 cm), despite accounting for respiratory motions.10
In this patient, an infra-diaphragmatic organ was lying in a supra-diaphragmatic location posing difficulty in estimating organ movement and applying planning target margins as referenced in the literature. In addition, the tumor extent was not clearly visible on the CT scan, posing a planning challenge in the absence of guidelines and standards for this type and location of tumor.
Hence, we employed 4DCT imaging, which demonstrated that internal target motion could be larger compared to that observed with esophageal cancer.7,8 With a 3D margin (Table 1), the PTV increased by 18.1%, resulting in higher OAR doses (Table 2).
CONCLUSION
Our study demonstrated that the tumor in the large hiatal hernia, which was displaced in the chest, can be effectively treated with chemoradiation therapy. It is, however, recommended that 4DCT be performed for target delineation to account for internal target motion, as the organ motion in this tumor does not represent that observed in patients with a GEJ tumor with normal anatomy. Our planning study demonstrated that VMAT helps minimize OAR doses. Furthermore, volumetric imaging is also recommended for larger interfractional motion, as seen in this case.
REFERENCES
- Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol. 2008;22:601-616.
- Namikawa T, Fukudome I, Munekage E, et al. Laparoscopy‐assisted distal gastrectomy for multiple adenocarcinomas in intrathoracic upside‐down stomach. Asian J Endosc Surg. 2016;9:57-60.
- Hagiwara C, Yajima K, Iwasaki Y, et al. Totally laparoscopic gastrectomy for early gastric cancer accompanied by huge hiatal hernia: a case report. Asian J Endosc Surg. 2016;9:61-64.
- Gandon A, Gronnier C, Renaud F, et al. Esophageal adenocarcinoma: impact of a large hiatal hernia on outcomes after surgery. Ann Surg. 2016;264:862-870.
- Crosby T, Hurt C, Falk S, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14:627-637.
- S Mukherjee, C Hurt, S Gwynne, et al. NEOSCOPE: a randomised phase II study of induction chemotherapy followed by either oxaliplatin/capecitabine or paclitaxel/carboplatin based chemoradiation as pre-operative regimen for resectable oesophageal adenocarcinoma. ASCO GI 2016, Oral Presentation. 2016.
- Zhao KL, Liao Z, Bucci MK, et al. Evaluation of respiratory-induced target motion for esophageal tumors at the gastroesophageal junction. Radiother Oncol. 2007;84:283-289.
- Guo YL, Li JB, Shao Q, Li YK, Zhang P. Comparative evaluation of CT-based and PET/4DCT-based planning target volumes in the radiation of primary esophageal cancer. Int J Clin Exp Med. 2015;8:21516-21524.
- Wand J, Lin SH, Dong L, et al. Quantifying the interfractional displacement of the gastroesophageal junction during radiation therapy for esophageal cancer. Int J Radiation Oncol Biol Phys. 2012;83(2)e273-280.
- Johnson ME, Pereira GC, El Naqa IM. et al. Determination of planning target volume for whole stomach irradiation using daily megavoltage computed tomographic images. Pract Radiat Oncol. 2012;2(4):e85-88.
Citation
Tambe N, Hingorani M, Beavis A, Dixit S. Radiation therapy planning for gastro-esophageal junctional carcinoma in a paraesophageal hiatal hernia. Appl Rad Oncol. 2018;(2):45-48 .
June 18, 2018
