Shingles After a Single Fraction of Radiation for Ewing Sarcoma
Images

Abstract
Reactivation of human herpes viruses is a feared complication for immunosuppressed cancer patients. We present the first reported case of varicella-zoster virus (VZV) reactivation after a single fraction of radiation. This 61-year-old woman began radiation therapy for Ewing sarcoma and within 24 hours complained of lower back pain in the dermatome of the dorsal root ganglion (DRG) that was within the radiation field. Antiviral therapy was initiated, and appropriate patient isolation was prioritized. Within 48 hours of radiation therapy (RT), vesicles erupted within the painful dermatome. All symptoms resolved following a course of valacyclovir, and no long-term neurologic symptoms were noted. We recommend that clinicians closely monitor patients receiving radiation therapy for symptoms of herpes zoster reactivation, provide swift and appropriate interventions when needed, and consider prophylactic antiviral treatment prior to cancer treatment.
Keywords: Radiation toxicity, varicella zoster, shingles, reactivation, side effects, immunosuppression, skin toxicity
Case Summary
A 61-year-old woman with a history of diffuse large B-cell lymphoma treated with R-CHOP for 6 cycles, and no evidence of disease for 10 years, presented to the local emergency department with progressive left lower extremity weakness. MR imaging showed an L4 vertebral body lesion with canal and foraminal stenosis, and bone biopsy revealed Ewing sarcoma. Dexamethasone therapy was initiated, and due to neurologic symptoms, RT was started upfront. Chemotherapy was added concurrently and continued following RT completion. One day after the radiation began, the patient noted lower back pain in a right-sided band-like distribution corresponding to the dermatome of the dorsal root ganglion (DRG) that was in the radiation field, with no vesicles or other dermatological lesions visible. She was started on valacyclovir 3 times daily due to concerns of VZV reactivation and an immunocompromised state. Two days after radiation began, right-sided lesions concerning for VZV were visible and appropriate isolation precautions were made. The diagnosis was visual and confirmed by PCR swab of the vesicular lesion. The patient has recovered without any postherpetic pain or scarring.
Imaging and Radiation Plans
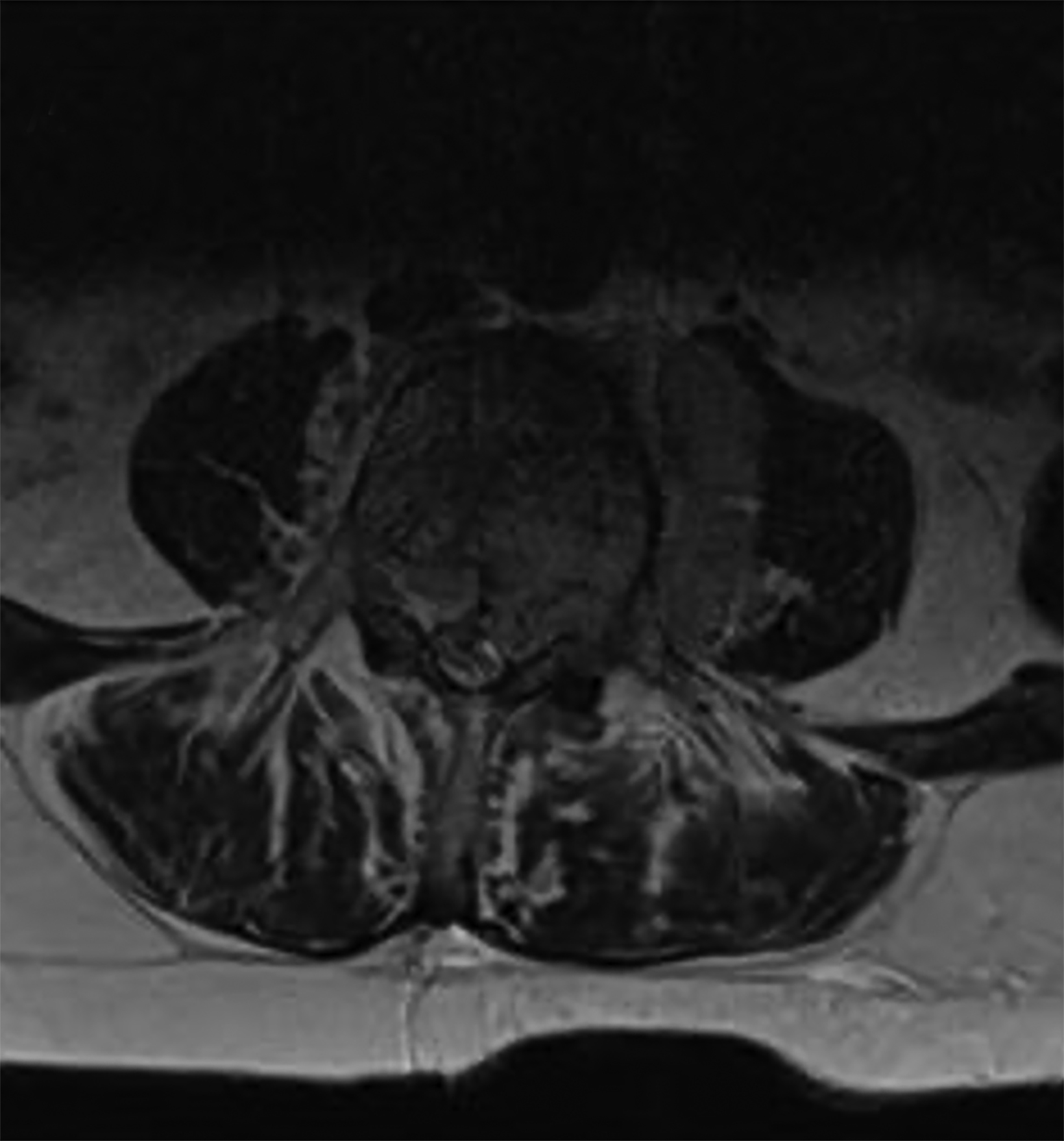
MRI lumbar spine was notable for diffuse neoplastic marrow replacement at L4, epidural neoplastic extension with moderate to severe canal stenosis, extraosseous extension into the left paraspinal space at L4 measuring 4.7 × 3.7 × 1.4 cm, abutment of the left psoas muscle, and encroachment into the left L4-5 neural foramen with severe stenosis (Figure 1).
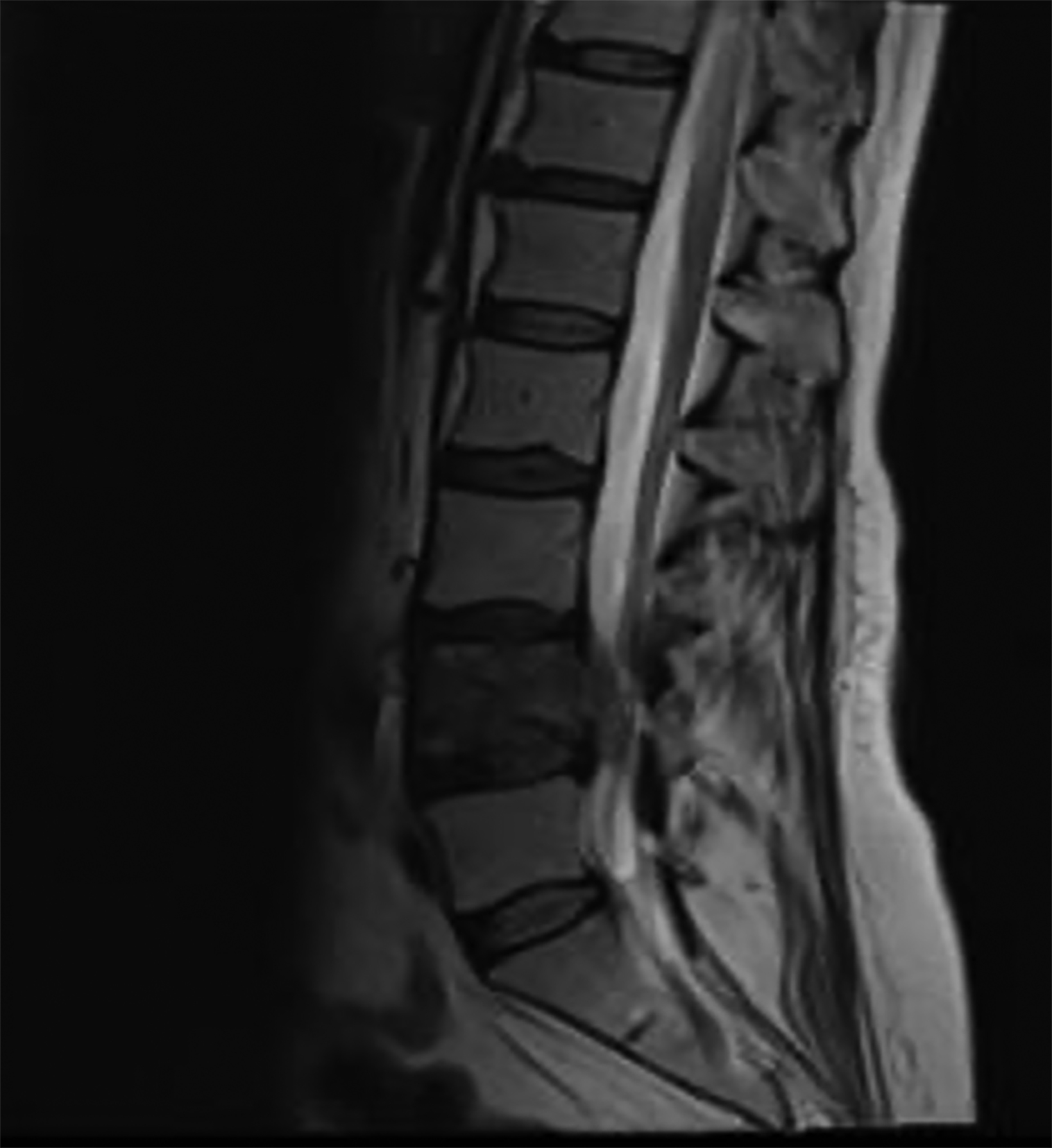
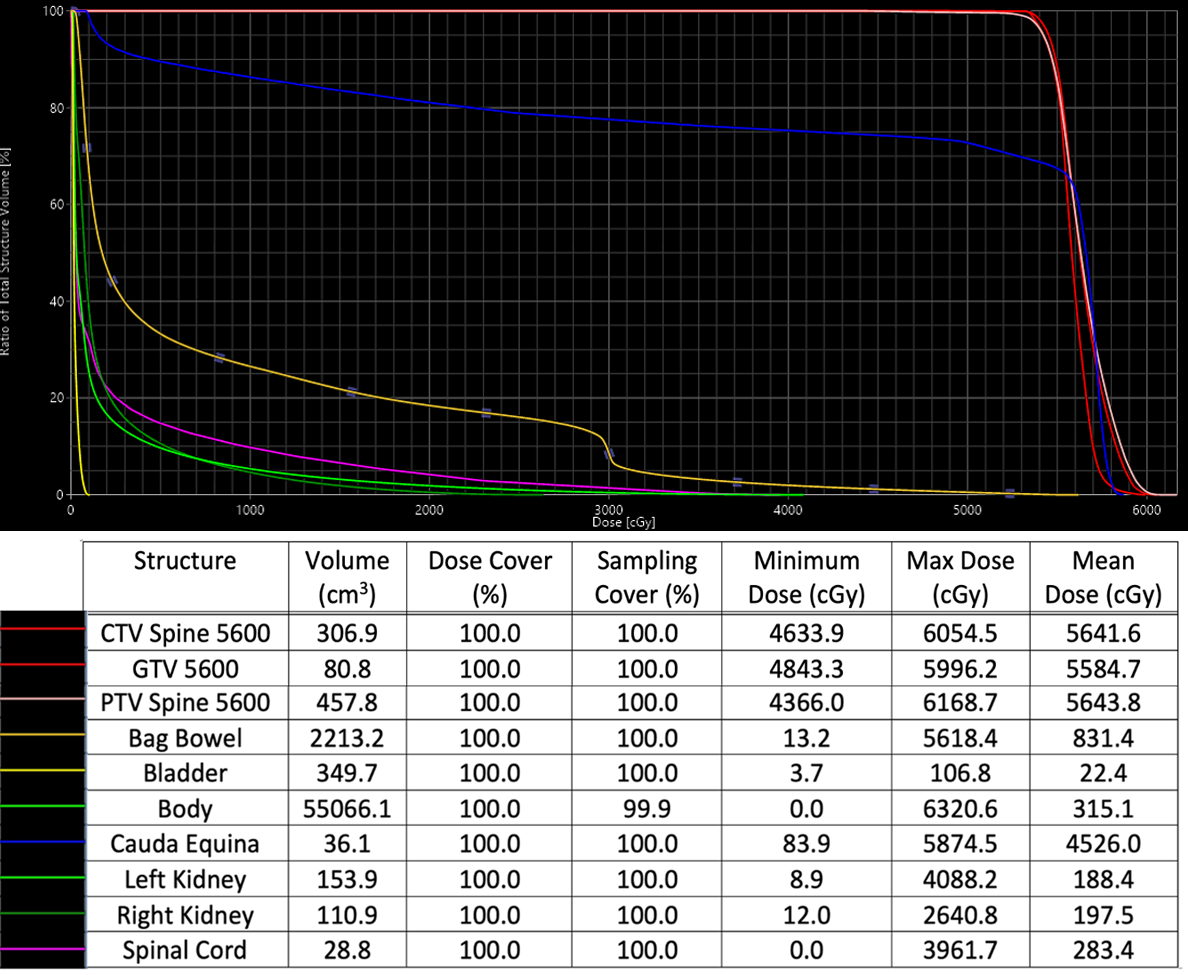
Due to neurologic symptoms, RT was started upfront and chemotherapy was added concurrently and continued following RT completion. Treatment consisted of a mixed proton/photon plan to a total of 56 Gy in 24 fractions. The initial plan was a simple 2-beam photon plan, anterior to posterior and posterior to anterior beams. For the final 12 fractions, the patient was switched to a proton plan with a posterior beam and a left posterior oblique beam to spare bowel toxicity (Figure 2). See Supplementary Figure 1 (available as a PDF in the online version of this article at www.appliedradiationoncology.com) for relevant dose statistics of the safe, effective, and peer-reviewed plan sum used to treat this patient.
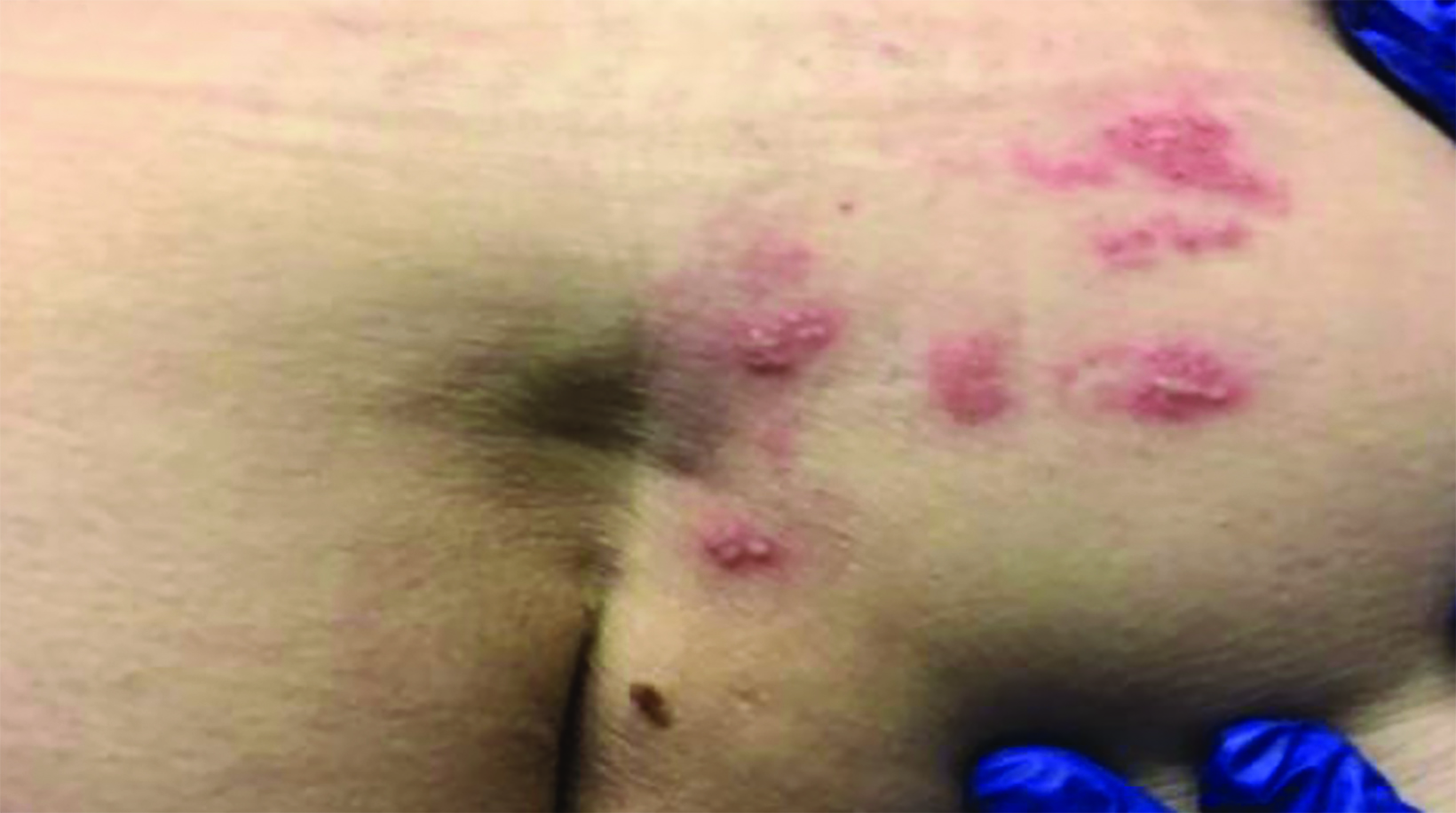
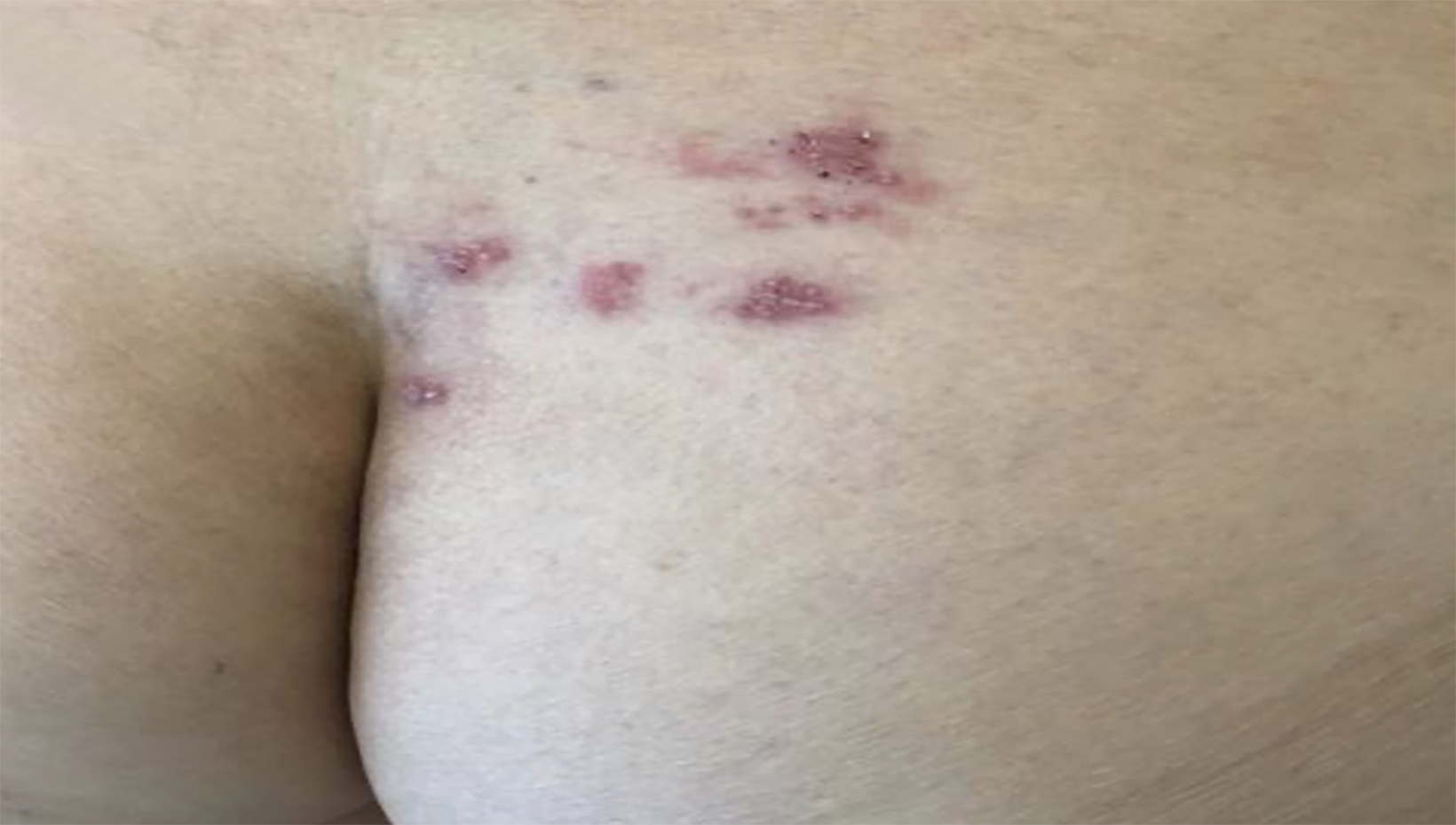
One day after the radiation began, the patient noted right-sided lower back pain. Within 48 hours, right-sided lesions concerning for VZV were visible and appropriate isolation precautions were made. Although there is variability regarding specific dermatomes, these vesicles were likely in the DRG of her L5/S1 dermatome, which corresponds to the area where she received radiation therapy. The lesions are seen in Figure 3.
Diagnosis
Physical examination and routine labs were otherwise unremarkable and there was no stigmata of VZV reactivation at this point. Possible diagnoses including atopic dermatitis, trauma, autoimmune dermatologic manifestations, and pain from musculoskeletal or neurogenic origin were considered and ruled out. The infectious diseases team was consulted and suspected VZV reactivation even prior to the appearance of lesions, particularly given the immunocompromised state of the patient. Of note, the patient received her second dose of the SHINGRIX (GlaxoSmithKline) vaccine in 2018 and had no prior history of shingles/VZV reactivation.
Discussion
Our patient is the first reported patient to have developed reactivation of an alphaherpesvirus varicella zoster virus (VZV) after only a single fraction of radiation.1 Reactivation of latent VZV, also known as herpes zoster or shingles, is most often seen with waning T cell immunity (eg, older or immunocompromised individuals). The first sign of herpes zoster is often superficial pain followed by a maculopapular rash in a unilateral dermatomal distribution that transitions into a vesicular rash. More concerning neurological complications include inflammation of the cranial nerves, encephalitis, meningitis, and significant ophthalmological complications. In addition, gastrointestinal manifestations such as pancreatitis and gastritis have been reported.2 Among the more concerning side effects, postherpetic neuralgia can develop in approximately 1 out of 5 patients who experience a Zoster outbreak.3 Those with postherpetic neuralgia experience significant decline in quality of life, and therapies such as tricyclic antidepressants, analgesics, and interventional therapies have notable drawbacks and often do not provide full relief.4,5 Therefore, it is best to ensure infection does not occur and to prioritize prevention, particularly in immunocompromised patients. It is important to note that previously reported cases of herpes zoster in cancer patients occurred much later in the course of radiation therapy or even months after completing treatment, and never after a single fraction of radiation therapy. Moreover, to our knowledge no direct investigation into the mechanism linking radiation therapy to alphaherpesvirus reactivation has been conducted. In this case report, we present a detailed overview of the case at hand, offer potential explanations, and discuss the implications of the clinical findings.
Both cancer as well as cancer treatments (including radiation therapy) have been shown to be independent risk factors for VZV reactivation. Multiple studies have demonstrated an increased risk of herpes zoster reactivation in breast cancer patients receiving radiation to the chest.6,7 Shimizuguchi et al reported a significantly increased incidence of herpes zoster in patients receiving radiation therapy over 5 years compared with those who did not receive radiation therapy (hazard ratio, 2.59, 95% CI, 1.84-3.66), leading the authors to recommend Shingrix vaccination and possible VZV prophylaxis prior to radiation therapy.8 Hodgkin’s lymphoma patients have demonstrated decreased cell-mediated immune response to VZV in vitro. Moreover, when treated with both radiation therapy and chemotherapy, these patients had decreased overall immune capacity compared with those treated with a single modality.9,10 Small-cell lung carcinoma patients have also experienced high occurrence of herpes zoster following combined radiation and chemotherapy.11
We postulate that in this scenario, the location of radiation therapy may have caused or lowered the threshold for a VZV outbreak. Since the radiation for this specific patient was directed near the spinal cord and VZV often lays latent in the sensory DRG,3 this may have increased risk for VZV reactivation. We suspect that the right lower lumbar/sacral DRG in this patient was harboring dormant virus.
Previous work has proposed mechanisms for how radiation therapy can reshape the tumor microenvironment, such as through inducing cell death and subsequently priming cytotoxic T lymphocytes to further attack tumor cells.12 Proposed mechanisms for radiation-therapy-induced immunosuppression leading to herpetic reactivation include immune dysfunction in the treatment region and disruption of the body’s ability to contain latent virus, both of which increase viral load and make reactivation more likely.13,14
As the patient had a known history of cancer and a nearby tumor prior to any reported clinical manifestations, we cannot rule out that the cause of VZV reactivation may have been the tumor microenvironment and that the timeline of radiation and VZV reactivation was coincidental. However, the symptoms of herpes zoster presented exclusively on the right side of the patient’s spine that was away from the left-sided tumor, making the tumor a less likely agent in the sequence of reactivation. Additionally, the VZV reactivation occurred only after radiation and was in the dermatome of the DRG corresponding to the precise target of radiation therapy.
We also note that although she was on concurrent steroids when this VZV outbreak occurred, the immunosuppression this caused was unlikely to have contributed to her Zoster outbreak, since she had taken multiple courses of steroids during prior cancer treatments and even during prior episodes of pain leading up to this hospital admission without such a complication. Moreover, while Qian et al showed that the incidence of VZV was higher in patients taking systemic corticosteroids, this increased incidence was mostly seen 1 to 3 months after initiation of the prolonged steroid regimen.15 Our patient showed reactivation after a single day of dexamethasone treatment, making dexamethasone an unlikely agent in VZV reactivation.
We postulate that there may be a common mechanism by which radiation therapy lowers the threshold for reactivation of latent alphaherpesviruses that has yet been to be elucidated, and that this mechanism may be distinct from immunosuppression. In addition, multiple factors may contribute to reactivation of VZV in patients receiving radiation therapy, such as the type of cancer, the type of radiation therapy provided, and the anatomical location of the radiation (eg, proximity to spinal cord). Ultimately, we can only posit that any perturbation may increase the likelihood of VZV reactivation.
VZV reactivation, which is typically dermatomal, can be associated with more severe disease or complications in immunocompromised patients, including those undergoing cancer treatment. Radiation oncologists must be vigilant when treating patients with a past history of VZV as the virus lies dormant and can seemingly be reactivated after a single dose of radiation therapy. In addition to VZV symptoms, complications of reactivation may hinder the patient’s cancer treatment.16 As a result, it is imperative that radiation oncologists closely monitor radiation therapy patients for VZV symptoms — especially if they have not received the Shingrix vaccine — and inform them about this possible side effect. Early detection and treatment can mitigate the course of reactivation. Given the relationship between viral load and likelihood of reactivation,14 there may be use for viral load estimation and/or VZV prophylaxis8 prior to patients undergoing radiation treatment. Future randomized clinical trials comparing the rates of VZV reactivation in radiation fields containing dorsal root ganglia and intervention in the form of antiviral prophylaxis would be a welcomed next step.
Conclusion
This interesting and concerning finding should spark more investigation into the pathogenesis of the reactivation of this disease in the setting of radiation therapy. Moreover, it is imperative to increase awareness among radiation oncologists of the possibility of alpha-herpes virus reactivation in their patients and to be more vigilant in screening patients who may be susceptible, as well as counseling patients on this rare but potentially serious side effect. Future clinical trials investigating the reliability of viral load estimation and/or VZV prophylaxis prior to radiation therapy could improve patient outcomes.
References
- Kennedy PG, Rovnak J, Badani H, Cohrs RJ. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-602. doi:10.1099/vir.0.000128. Epub 2015 Mar 20.
- John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826. doi:10.1016/j.idc.2017.07.016.
- Macomber MW, Mullane KM, Liauw SL. Herpes zoster and radiation therapy: what radiation oncologists need to know about diagnosing, preventing, and treating herpes zoster. Pract Radiat Oncol. 2014;4(1):58-64. doi:10.1016/j.prro.2013.02.004.
- Lin CS, Lin YC, Lao HC, Chen CC. Interventional treatments for postherpetic neuralgia: a systematic review. Pain Physician. 2019;22(3):209-228.
- Hadley GR, Gayle JA, Ripoll J, et al. Post-herpetic neuralgia: a review. Curr Pain Headache Rep. 2016;20(3):17. doi: 10.1007/s11916-016-0548-x. Erratum in: Curr Pain Headache Rep. 2016;20(4):28.
- Ellis, F. Herpes zoster after irradiation. Br Med J. 1949;2(4640):1323-1328. doi:10.1136/bmj.2.4640.1323
- Lai Y et al. Increased risk of Varicella-Zoster Virus infection in patients with breast cancer after adjuvant radiotherapy: a population-based cohort study. PLoS ONE. 2019;14(1):1-10. doi:10.1371/journal.pone.0209365
- Shimizuguchi T, Sekiya N, Hara K, et al. Radiation therapy and the risk of Herpes zoster in patients with cancer. Cancer. 2020;126(15):3552-3559. doi:10.1002/cncr.32926
- Ruckdeschel JC, Schimpff SC, Smyth AC, Mardiney Jr. MR. Herpes zoster and impaired cell-associated immunity to the Varicella-zoster Virus in patients with Hodgkin’s disease. Am J Med. 1977;62(1):77-85.
- Guinee VF et al. The incidence of Herpes zoster in patients with Hodgkin’s disease. An analysis of prognostic factors. Cancer. 1985;56(3):642-648.
- Feld R, Evans WK, DeBoer G. Herpes zoster in patients with small-cell carcinoma of the lung receiving combined modality Treatment. Ann. Intern Med. 1980;93(2):282–283. doi:10.7326/0003-4819-93-2-282
- Ruckert M, Flohr A, Hecht M, Gaipl US. Radiotherapy and the immune system: more than just immune suppression. Stem Cells. 2021;39(9):1155-1165.
- Ruocco V, Ruocco E, Piccolo V, Brunetti G, Guerrera LP, Wolf R. The immunocompromised district in dermatology: a unifying pathogenic view of the regional immune dysregulation. Clin Dermatol. 2014;32(5):569-576.
- Schiffer JT, Corey L. Rapid host immune response and viral dynamics in herpes simplex virus-2 infection. Nat. Med. 2013;19(3):280-290.
- Qian J, Banks E, Macartney K, Heywood AE, Lassere MN, Liu B. Corticosteroid use and risk of Herpes zoster in a population-based cohort. Mayo Clinic Proceed. 2021;96(11):2843-2853. doi:10.1016/j.mayocp.2021.05.029
- Ramirez-Fort MK, Zeng J, Feily A, et al. Radiotherapy-induced reactivation of neurotrophic human herpes viruses: overview and management. J Clin Vi- rol. 2018;98:18-27.
Citation
V A, S D, M D, I L, I P, K C. Shingles After a Single Fraction of Radiation for Ewing Sarcoma. Appl Radiat Oncol. 2022;(3):35-38.
October 14, 2022