Special Considerations of Pelvic Radiation Therapy in the Adolescent and Young Adult (AYA) Female Population
Introduction
The adolescent and young adult (AYA) cancer population (generally defined as patients ages 15-39) represents an important group of patients with unique needs compared with the pediatric or older adult populations. The National Cancer Institute estimates that almost 86,000 AYA patients are diagnosed with cancer each year, which represents approximately 4% of all cancer diagnoses.1 The incidence of cancer in this population has also been rising over the last decade, with a 0.3% increase each year from 2010 to 2019, for reasons not well understood at this time.1 The survival rates of AYA patients have also not incrementally improved over the last decade compared with other cancer populations.1 Unique challenges identified in this population include delays in initial diagnosis, decreased access to and participation in clinical trials, differences in tumor biology, poorer compliance and adherence to prescribed therapies, lack of communication and resources to address their specific psychosocial needs, negative impact of therapy on body image and sexuality, loss of fertility, and financial toxicity of treatment (
Delays in the initial diagnosis Decreased access to and participation in clinical trials Differences in tumor biology Poorer compliance and adherence to prescribed therapies Lack of communication and resources to address specific psychosocial needs Negative impact of therapy on body image and sexuality Loss of fertility Financial toxicity of treatment |
Fertility and Premature Ovarian Insufficiency
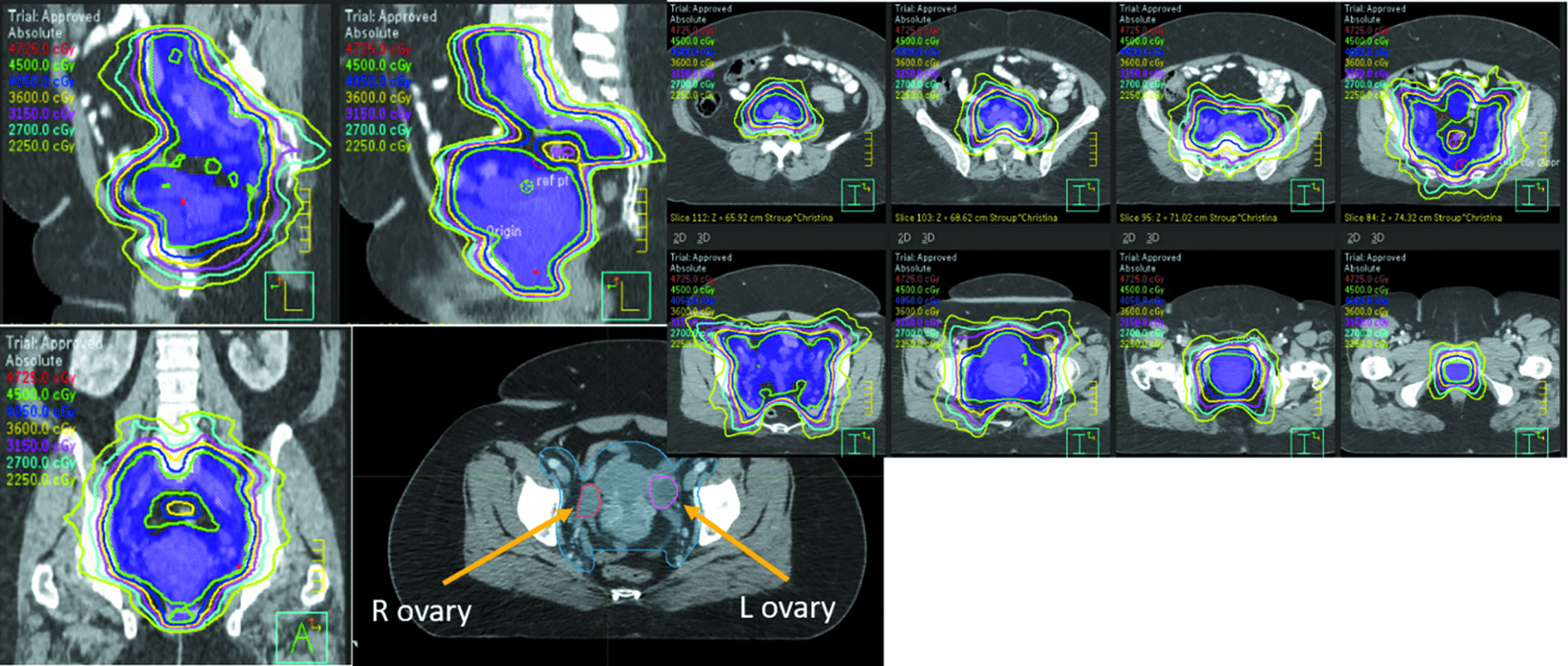
The late side effect that most commonly comes to mind when thinking about AYA patients is loss of fertility. Approximately 50% of cervical cancer patients are premenopausal at diagnosis and 15% of rectal cancer patients are under the age of 50 at diagnosis.4 Even low doses of pelvic RT can cause a total shutdown of ovarian function in women of this age group, with doses of less than 4 Gy (and even less than 2 Gy) associated with the elimination of 50% of the oocyte pool.5 Standard pelvic dosing of 45-50 Gy (
A typical pelvic radiation therapy plan using intensity-modulated radiation therapy, with ovaries identified on CT.
Loss of Viable Eggs
The loss of viable eggs after low doses of pelvic RT leads to an inability to create embryos later in life. If it is clinically safe to delay cancer treatment, patients should be urgently referred to specialists in reproductive endocrinology and infertility (REI) for counseling and discussion of fertility options. These typically include cryopreservation of embryos (if the patient has a partner or sperm donor they would like to create embryos with), cryopreservation of oocytes, and cryopreservation of ovarian tissue.6 Data are limited, but according to one retrospective study from the University of Southern California, thawed oocytes had a lower survival rate than embryos (79.1% vs 90.1%), but similar rates of fertilization (76.2% vs 72.8%) and live birth rates (25% in both groups).7 Another study from New York University showed a live birth rate of 39% from thawed oocytes, with the best outcomes seen in patients less than 38 years old and a higher number of thawed oocytes.8 Cryopreservation of ovarian tissue led to a live birth rate of 25.0% in a large registry study from the Netherlands, with increased rates of success in women less than 35 years of age at the time of ovarian tissue freezing (28.2%) vs greater than 35 years of age at the time of freezing (16.7%).9 It is also important to have a discussion of the financial aspects of these preservation procedures, as they are not typically covered by insurance. A limited number of national programs can help provide funding to offset costs, but the upfront cost and ongoing costs of cryotherapy storage can be cost-prohibitive to many AYA patients.
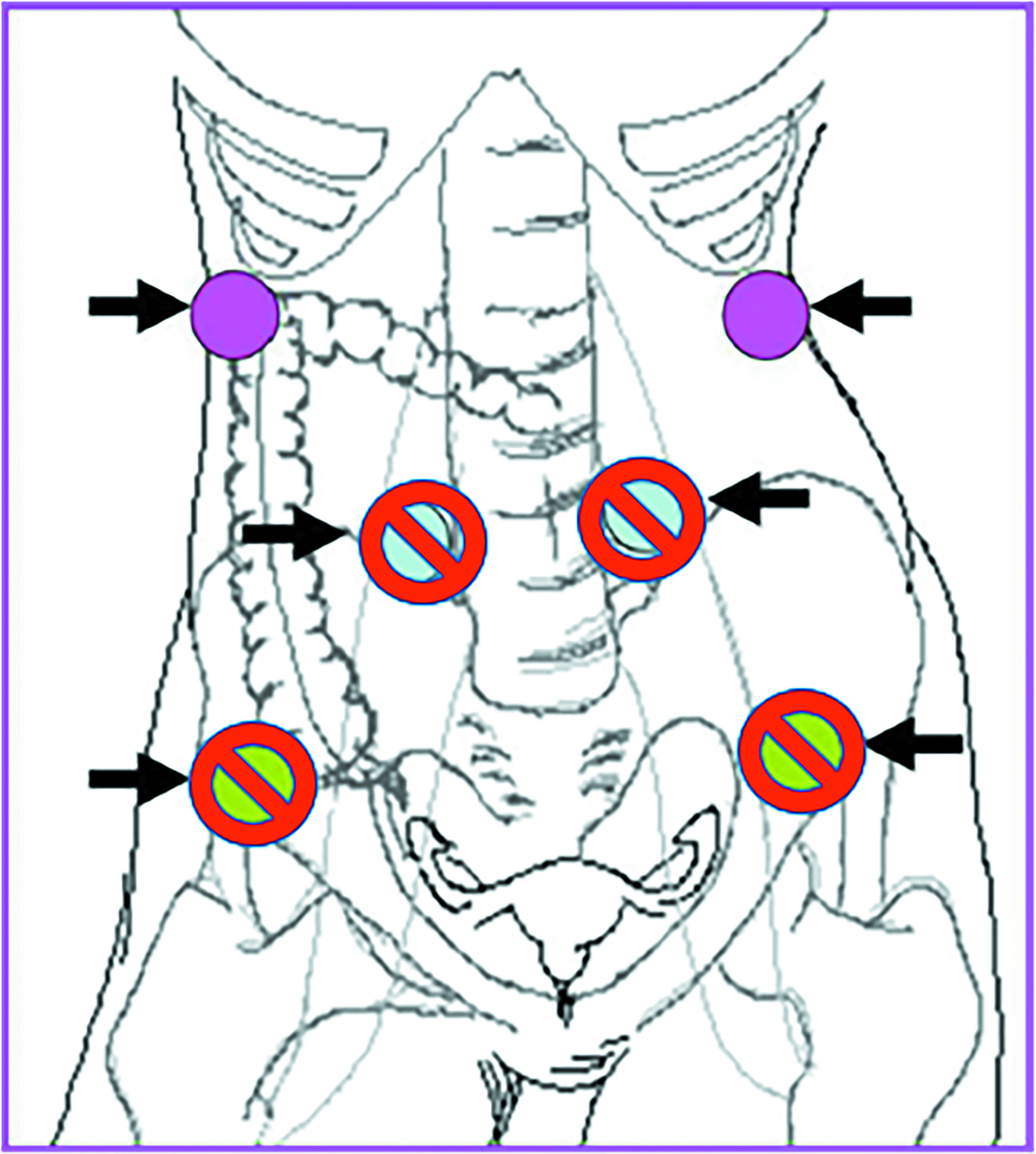
Another option for patients who do not have ovarian involvement by tumor is surgical ovarian transposition.10 With this procedure, the ovary is surgically transposed with its vascular pedicle to another location, ideally well above the pelvic brim, to minimize the radiation dose to the ovary. The procedure is most often performed laparoscopically, allowing for relatively quick recovery times. Transposition can be performed concurrently with other surgical procedures (such as pelvic node debulking, para-aortic nodal sampling, diverting ostomies, or hysterectomy/trachelectomy) or as stand-alone surgery. The ovaries should ideally be transposed at least 3 cm above the upper border of the radiation field, well above the pelvic brim and as lateral as possible (
Ideal location of transposed ovaries that are high and lateral in the abdomen (pink). Less ideal locations are also shown (crossed out).
CT simulation can typically be performed within a week of the procedure if the abdominal wall has sufficiently deflated (after surgical insufflation with laparoscopy) for reproducible treatment planning and delivery. The surgeon should mark the location of the transposed ovary or ovaries with a surgical clip and the ovarian tissue should be contoured for dosimetric evaluation. If the ovary is high enough in the abdomen, there should be minimal direct dosing to the ovary; however, the ovary will likely still receive some radiation exposure via internal scatter. This is important to explain when counseling patients, as the risk of ovarian failure remains given the tissue’s sensitivity to radiation. Other risks associated with ovarian transposition include complications at the time of surgery, ovarian torsion, vascular injury, fallopian tube infarction, and small bowel obstruction due to postsurgical adhesions. Ovarian cyst formation is common and reported in up to 95% of patients but is unlikely related to the transposition procedure. Patients who undergo successful ovarian transposition with function retained after radiation therapy may retain viable eggs after treatment, which can later be retrieved for in vitro fertilization procedures. Ovarian transposition tends to be more successful in younger women, with the best outcomes seen in patients under age 35 (preservation rates by age: 25-30: 87.5%; 31-35: 62.5%; and 35-40: 42.9%).17 National guidelines by the American Society of Clinical Oncology and the National Cancer Comprehensive Network3,18 both recommend offering ovarian transposition to appropriately selected AYA cancer patients (
| GOOD CANDIDATES FOR TRANSPOSITION | POOR CANDIDATES FOR TRANSPOSITION |
|---|---|
Premenopausal Patients who prefer not to take long-term hormone replacement Best outcomes in patients aged < 35-40 Low risk of ovarian metastases from primary cancer Medically operable NOT receiving gonadotoxic chemotherapy |
Perimenopausal/postmenopausal High risk of ovarian metastases from primary cancer Imaging can be important to determine the extent of the disease prior to offering the procedure Medically inoperable Receiving gonadotoxic chemotherapy |
Premature Ovarian Insufficiency
Although fertility is an important consideration, the implications of POI or premature menopause on a young woman’s health can often be overlooked by providers. Premature ovarian insufficiency is age dependent, with doses of 16.5 Gy at age 20 vs 10 Gy at age 45 to deplete the ovarian oocyte pool with conventional fractionation of 1.8-2.0 Gy per fraction. The shutdown of ovarian tissue with low doses of RT leads to decreased production of estrogen. Estrogen has many important normal functions in the body unrelated to reproductive health, including maintaining bone mineral density, decreasing the risk of cardiovascular disease by lowering the risk of atherosclerosis, neuroprotective effects due to estrogen-dependent DNA repair mechanisms in the brain, as well as effects on cognition and the immune system. The decreased lifetime exposure to estrogen in AYA patients can therefore have other long-term consequences on their overall health and life expectancy.
Menopause also comes with a host of symptoms that can substantially alter the quality of life and affect the body image of AYA patients. These menopausal symptoms include vasomotor symptoms (hot flashes), vaginal changes and decreased libido (see “Sexual Health”), depressed mood/anxiety/mental health changes from the rapid loss of estrogen, and memory changes. Many of these symptoms affect a patient’s ability to work and can significantly impact their relationships at a time in life when many patients are building their careers and family lives.
There are treatment options for patients who undergo POI and referral should be made early to a women’s health specialist, ideally someone who has more familiarity with POI due to cancer therapies. Options may include hormone replacement (in patients with nonhormonally driven cancers), symptomatic management for hot flashes (eg, venlafaxine, fezolinetant), and antidepressants/antianxiolytics. Patients may also benefit from a referral to a behavioral health specialist. Another option is to prevent POI by performing ovarian transposition prior to pelvic RT. Even if a patient does not desire fertility preservation in the future, successful transposition and protection of the ovary can allow for continued endogenous estrogen production in appropriately selected patients
Uterine Changes
The uterus is more resistant to radiation-induced changes than the ovaries; however, at the doses most typically used for gynecologic cancers, colorectal cancers, and sarcomas, long-term vascular changes and fibrosis are common. The decreased vascularity in the uterus translates into an inability to increase blood flow sufficiently to support the gestation of a fetus. The fibrosis that occurs in the wall of the uterus means that the uterus cannot expand as it normally would support a pregnancy.19 Radiation to the uterus can lead to infertility, an increased risk of miscarriage, low-birth-weight infants, and premature births. It is important to counsel a patient that even if they choose to undergo cryopreservation or ovarian transposition and can create embryos in the future, depending on the dose of radiation that the uterus received, they may be unable to carry their own pregnancy and may require the use of a gestational carrier.20 The use of a gestational carrier comes with the potential for considerable financial cost, as well as possible state-dependent legal implications, which can significantly affect a patient and her family planning and decision-making. If a patient would like to pursue carrying her own pregnancy, referral should be made to maternal-fetal medicine providers for counseling and care.
Sexual Health
Pelvic RT can have significant effects on a patient’s sexual health.21 Studies suggest that patients receiving pelvic RT have significantly lower scores of satisfaction with social support and sexual function.22 Since many AYA patients are often in a stage of trying to understand their sexuality and build and maintain relationships at the time of their cancer diagnosis, treatment that affects their sexual health can have a more devastating impact on body image, relationships, and sexual enjoyment.23 Unfortunately, female patients routinely rate communication surrounding sexual function changes by physicians to be inadequate. A small study from 202324 found that patients felt that (1) they were not properly informed about sexual side effects and felt “blindsided and embarrassed,” (2) they were psychologically affected with lower self-esteem and no longer feeling sexy, (3) they were not supported by their physicians and frequently had to turn to the internet for information and community support, and (4) their radiation oncologist could be more proactive in asking about sexual history and identifying individual patient priorities surrounding sexual health after treatment.
Pelvic radiation therapy primarily impacts vulvovaginal health and libido. Vaginal changes after pelvic RT and/or brachytherapy can include vaginal dryness, fibrosis, stenosis, vaginal length shortening, loss of elasticity, and vaginal closure. The reported incidence of vaginal stenosis ranges from 2.5% to 88% depending on a multitude of factors, including cancer type, RT dose, and the volume of vagina treated. Vulvar changes after pelvic RT can include painful labial adhesions and labial fibrosis. Patients may also experience dyspareunia and painful pelvic floor symptoms due to pelvic floor spasms. Patients should be counseled on these potential late effects at the time of consultation, as well as during and after treatment.
Vaginal dilators (
Examples of silicone and hard plastic vaginal dilators in different sizes.

Patients should be counseled on how to appropriately use the dilator, lubrication (coconut oil, KY Jelly, or other vaginal moisturizers are recommended), and frequency of use. Dilation should start 4-6 weeks after completion of RT and should likely continue long-term (vaginal stenosis typically occurs 3-5 y after treatment), although no data or consensus exist as to the optimal duration of therapy.32 Based on a large survey of US-based radiation oncologists, most providers recommend dilating three times per week for 5-10 minutes per session.25 Vaginal intercourse may be difficult and painful for patients after treatment, but many patients may eventually have more comfortable vaginal intercourse with dilator use. Ongoing counseling and assessment of a patient’s sexual health and vaginal tissue by providers can help improve patient compliance with dilator use and increase patient satisfaction and outcomes.32 Some patients may also benefit from topical hormonal therapy.26 In patients who have difficulty with dilator use or labial/vaginal adhesions, referral should be made to a urogynecologist for further assessment, as surgical treatment may be needed. Many patients also benefit from a referral for pelvic floor physical therapy to help with pelvic floor laxity (which can lead to incontinence symptoms, in addition to sexual health changes) or pelvic floor tightness/spasms, which can lead to dyspareunia and vaginismus symptoms.
Decreased libido can result from POI as discussed but can also be caused by diminished views of body image after cancer diagnosis and treatment, decreased sexual enjoyment due to pain/discomfort from vulvovaginal changes, or depression.23 Patients should be referred to behavioral health specialists and sexual therapists when appropriate.26 Sexual therapy can help patients discover new ways to experience intimacy and sexual enjoyment, leading to improved relationships and quality of life in AYA patients.
Financial Toxicity
The cost of a cancer diagnosis can be immense and cause significant financial impact and harm to AYA patients who are just starting to build careers and wealth. Patients who have financial toxicity also have inferior outcomes.33 Direct cancer care-related costs include those of health insurance; copays for appointments, treatments, and prescribed medications; over-the-counter medications recommended for symptom management; and daily transportation costs. Additional costs may also be related to travel, housing, and meals. Indirect costs include time away from school/college and loss of work hours for patients and caregivers.
There are also considerable costs to being a cancer survivor. Approximately 80% of AYA cancer patients are long-term survivors2 and face significant ongoing costs of maintaining good health insurance with a preexisting condition, copays for follow-up cancer surveillance tests and appointments, in addition to the costs associated with seeing other providers/specialists for the treatment of long-term side effects of cancer treatments. These appointments lead to continued days of missed school and work for patients and caregivers.
Acknowledging the financial impact of a cancer diagnosis and, more specifically, how multiple weeks of daily pelvic RT can affect a patient and her family is an important step in helping patients find resources to manage costs. A recent large cross-sectional study of 1075 patients from Germany who underwent RT revealed that the overall prevalence of financial toxicity was higher than anticipated with subjective financial distress reported by 41% of patients.34 Prasad et al published a financial toxicity screening tool for radiation oncology that can help identify early-onset, patient-reported financial toxicity after radiation therapy with three variables: age, money owed, and copayment-related concerns.35 Such tools can help radiation oncologists more easily identify at-risk patients and help provide appropriate resources. Social workers can be helpful in identifying financial grants and funds that a patient may be eligible for to help defray expenses, as well as helping to identify resources for transportation and housing that insurance or other cancer organizations may help cover. Financial navigators and advocates can assist patients in better understanding and managing treatment-related bills, in addition to helping patients with payment plans that they can more easily manage. The medical team can also consider more hypofractionated treatment courses (when clinically appropriate) and support patients with the appropriate letters and paperwork to help them maintain their jobs while undergoing treatment.
Psychological Impact
A cancer diagnosis has a psychological impact at any age, but a cancer diagnosis in AYA patients can be even more psychologically devastating since the cancer diagnosis and treatment are occurring at a major time for living independent adult lives and building careers and relationships.
After diagnosis, cancer treatments and their short- and long-term side effects can compound the psychological impact. In addition to the side effects discussed, bowel and urinary changes caused by pelvic radiation therapy can have a major psychological impact (see the accompanying article in this issue, “Late Effects of Pelvic Radiation Therapy”). Adams et al surveyed 418 patients who had received pelvic RT 1-11 years previously and measured patient-reported toxicity, psychological morbidity, and quality of life. Female patients reported moderate/severe toxicity with bowel (59% of patients), urine urgency (49% of patients), and the ability to have a sexual relationship (24% of patients). These symptoms were just as frequent 6-11 years after RT compared with 1-5 years after RT, and symptom severity was associated with poorer quality of life and higher levels of depression.36
Unfortunately, women treated with pelvic RT routinely report unmet post-treatment psychosexual and psychological needs. It is therefore important to discuss these side effects at each follow-up appointment and support patients with resources. A randomized controlled trial showed that patients who received a psychosexual rehabilitation booklet over standard information were more likely to have higher dilator adherence and be better educated on psychosexual side effects and rehabilitation options.37 Giving patients tools and information after pelvic RT treatment can help reduce distress and positively affect patients psychologically.
Referral to specific AYA cancer programs can be helpful, if available. These programs typically help patients navigate their diagnosis, testing, and appointments. They are also connected to AYA-specific resources, including behavioral health specialists who can help patients better weather the psychological storm. If an AYA program is not available, early intervention by a social worker or other behavioral health specialist can be instrumental in helping patients access care and increase compliance with treatment. In addition, partners, children, and other caregivers are often negatively impacted. Family counselors, support groups, and psycho-oncologists can help patients and their families in survivorship as well. Proactive support of patients’ and their caregivers’ mental health during diagnosis, treatment, and survivorship can help create a better therapeutic relationship through the care continuum and improve the quality of life of AYA patients.
Survivorship: Other Considerations
Adolescent and young adult patients should also be counseled and monitored appropriately for late side effects that may affect all patients undergoing pelvic RT, including late bladder and bowel changes (eg, incontinence, functional impairment, cystitis/proctitis), bone and joint changes, and surveillance for secondary malignancies. Referrals should be made to appropriate specialists, if indicated, in reproductive health (REI), maternal-fetal medicine, urogynecology/urology, gastroenterology/colorectal surgery, physical therapy (pelvic floor, lymphedema, functional strength/mobility), behavioral health, and others, to help ensure better long-term quality of life for these young survivors.
Conclusion
Female AYA patients can experience significant impact on their physical and mental health with pelvic RT, including loss of fertility, POI, sexual health changes, financial toxicity, and psychological tolls. Awareness of these effects and available resources can help radiation oncology providers to better communicate and counsel patients, set expectations for the long term, and proactively manage these effects in a vulnerable population, which can positively impact long-term survivorship and quality of life.
References
- National Cancer Institute. Adolescents and young adults with cancer was originally published by the National Cancer Institute. 2023. Accessed June 10, 2023.
- Coccia PF. Overview of adolescent and young adult oncology. J Oncol Pract. 2019; 5(15):235-237
- Bhatia S. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Adolescent and Young Adult (AYA) Oncology V.2.2024. National Comprehensive Cancer Network, Inc. All rights reserved. Accessed June 10, 2023. www.NCCN.org.
- Group. USCSW. U.S. Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2023. Accessed June 15, 2023. https://www.cdc.gov/cancer/dataviz
- Wallace WHB, Thomson AB, Kelsey TW. The radiosensitivity of the human Oocyte. Hum Reprod. 2003; 1(18):117-121
- Wallace WHB, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered. Lancet Oncol. 2005; 4(6):209-218
- Ho JR, Woo I, Louie KA. comparison of live birth rates and perinatal outcomes between cryopreserved oocytes and cryopreserved embryos. J Assist Reprod Genet. 2017; 10(34):1359-1366
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022; 1(118):158-166
- Lotz L, Bender-Liebenthron J, Dittrich R. Determinants of transplantation success with cryopreserved ovarian tissue: data from 196 women of the FertiPROTEKT network. Hum Reprod. 2022; 12(37):2787-2796
- Moawad NS, Santamaria E, Rhoton-Vlasak A, Lightsey JL. Laparoscopic ovarian transposition before pelvic cancer treatment: ovarian function and fertility preservation. J Minim Invasive Gynecol. 2017; 1(24):28-35
- Hoekman EJ, Broeders E, Louwe LA. Ovarian function after ovarian transposition and additional pelvic radiotherapy: a systematic review. Eur J Surg Oncol. 2019; 8(45):1328-1340
- Buonomo B, Multinu F, Casarin J. Ovarian transposition in patients with cervical cancer prior to pelvic radiotherapy: a systematic review. Int J Gynecol Cancer. 2021; 3(31):360-370
- Swift BE, Leung E, Vicus D, Covens A. Laparoscopic ovarian transposition prior to pelvic radiation for gynecologic cancer. Gynecol Oncol Rep. 2018;24:78-82. doi:10.1016/j.gore.2018.04.005
- Morice P, Juncker L, Rey A. Ovarian transposition for patients with cervical carcinoma treated by radiosurgical combination. Fertil Steril. 2000; 4(74):743-748
- Bisharah M, Tulandi T. Laparoscopic preservation of ovarian function: an underused procedure. Am J Obstet Gynecol. 2003; 2(188):367-370
- Yin L, Lu S, Zhu J, Zhang W, Ke G. Ovarian transposition before radiotherapy in cervical cancer patients: functional outcome and the adequate dose constraint. Radiat Oncol. 2019;14(1):100. doi:10.1186/s13014-019-1312-2
- Hoekman EJ, Knoester D, Peters AAW. Ovarian survival after pelvic radiation: transposition until the age of 35 years. Arch Gynecol Obstet. 2018; 5(298):1001-1007
- Oktay K, Harvey BE, Partridge AH. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018; 19(36):1994-2001
- Critchley HOD, Wallace WHB. Impact of cancer treatment on uterine function. J Natl Cancer Inst Monogr. 2005;34:64-68. doi: 10.1093/jncimonographs/lgi022
- Teh WT, Stern C, Chander S, Hickey M. The impact of uterine radiation on subsequent fertility and pregnancy outcomes. Biomed Res Int. 2014; 2014:482968. doi:10.1155/2014/482968
- Jensen PT, Froeding LP. Pelvic radiotherapy and sexual function in women. Transl Androl Urol. 2015; 2(4):186-205
- Rodrigues AC, Teixeira R, Teixeira T. Impact of pelvic radiotherapy on female sexuality. Arch Gynecol Obstet. 2012; 2(285):505-514
- Savoie MB, Paciorek A, Van Loon K. Sexual function remains persistently low in women after treatment for colorectal cancer and anal squamous cell carcinoma. J Sex Med. 2023; 4(20):439-446
- Morgan O, Schnur J, Caban-Martinez AJ. A qualitative analysis of female patient perspectives on physician communication regarding sexual dysfunction associated with pelvic radiotherapy. J Sex Med. 2023; 6(20):813-820
- Kachnic LA, Bruner DW, Qureshi MM, Russo GA. Perceptions and practices regarding women’s vaginal health following radiation therapy: a survey of radiation oncologists practicing in the United States. Pract Radiat Oncol. 2017; 5(7):356-363
- Carter J, Lacchetti C, Andersen BL. Interventions to address sexual problems in people with cancer: American society of clinical oncology clinical practice guideline adaptation of cancer care Ontario guideline. J Clin Oncol. 2018; 5(36):492-511
- Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;2014(9):CD007291. doi:10.1002/14651858.CD007291.pub2
- Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;2014(9):CD007291. doi:10.1002/14651858.CD007291.pub3
- Martins J, Vaz AF, Grion RC, Costa-Paiva L, Baccaro LF. Correction to: topical estrogen, testosterone, and vaginal dilator in the prevention of vaginal stenosis after radiotherapy in women with cervical cancer: a randomized clinical trial. BMC Cancer. 2021;21(1):811. doi:10.1186/s12885-021-08543- 8
- Briere TM, Crane CH, Beddar S. Reproducibility and genital sparing with a vaginal dilator used for female anal cancer patients. Radiother Oncol. 2012; 2(104):161-166
- Son CH, Law E, Oh JH. Dosimetric predictors of radiation-induced vaginal stenosis after pelvic radiation therapy for rectal and Anal cancer. Int J Radiat Oncol Biol Phys. 2015; 3(92):548-554
- Damast S, Jeffery DD, Son CH. Literature review of vaginal stenosis and dilator use in radiation oncology. Pract Radiat Oncol. 2019; 6(9):479-491
- Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018; 2(68):153-165
- Fabian A, Rühle A, Domschikowski J. Financial toxicity in cancer patients undergoing radiotherapy in a universal health care system - A prospective multicenter study of 1075 patients. Radiother Oncol. 2023;183:109604. doi:10.1016/j.radonc.2023.109604
- Prasad RN, Patel TT, Keith SW. Development of a financial toxicity screening tool for radiation oncology: a secondary analysis of a pilot prospective patient-reported outcomes study. Adv Radiat Oncol. 2021;6(6):100782. doi:10.1016/j.adro.2021.100782
- Adams E, Boulton MG, Horne A. The effects of pelvic radiotherapy on cancer survivors: symptom profile, psychological morbidity and quality of life. Clin Oncol (R Coll Radiol). 2014; 1(26):10-17
- Lubotzky FP, Butow P, Hunt C. A psychosexual rehabilitation booklet increases vaginal dilator adherence and knowledge in women undergoing pelvic radiation therapy for gynaecological or anorectal cancer: a randomised controlled trial. Clin Oncol (R Coll Radiol). 2019; 2(31):124-131
Citation
SR A. Special Considerations of Pelvic Radiation Therapy in the Adolescent and Young Adult (AYA) Female Population. Appl Radiat Oncol. 2023;(3):5-12.
doi:10.37549/ARO-D-23-00017
September 1, 2023