Stent Migration and Fistula Formation Following Stereotactic Body Radiation Therapy for Pancreatic Cancer
Affiliations
- 1 University of South Florida, Tampa, FL
- 2 University of South Florida Morsani College of Medicine, Tampa, FL
- 3 Moffitt Cancer Center, Center Tampa, FL
Locally advanced pancreatic cancer (LAPC) is associated with a poor prognosis, with rates of eventual surgical resection after neoadjuvant therapies ranging from 10% to 30%. When such tumors are in the pancreatic head, obstructive jaundice is often the presenting symptom, necessitating endoscopic stent placement. Interval stent migration is possible at any time due to several factors, including the mechanical properties of self-expandable metal stents (SEMS), complete sphincterotomy, tumor regrowth, and improved tumor response from chemotherapy and radiation. A 75-year-old woman presented with pancreatic head/neck LAPC who received chemotherapy and stereotactic MR-guided adaptive radiation therapy (SMART) with ablative dose on an MRI linear accelerator. Post-treatment imaging at 6 months incidentally revealed that the previously placed biliary SEMS was dislodged into the duodenum. Endoscopic removal was not performed due to a 10-mm fistulous opening proximal to the major papilla in direct communication with the stent. This case reports stent migration and fistula formation postablative SMART.
Case Summary
A 75-year-old woman presented with obstructive jaundice to her primary care physician and was sent to the emergency room for further evaluation. Ultrasound was performed, which showed dilation of the common bile duct, and a proceeding MR cholangiopancreatography displayed a solid mass in the head of the pancreas with a double-duct sign and a CA 19-9 of 1036 U/mL. The patient underwent an endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatogram (ERCP). Fine-needle biopsy confirmed pancreatic adenocarcinoma. She tolerated 4 cycles of chemotherapy (FOLFIRINOX), starting from February 2022 well, and the CA 19-9 declined to 526 U/mL. She then received stereotactic MR-guided adaptive radiation therapy (SMART) to a dose of 50 Gy in 5 fractions with staging done on August 3, 2022, and treatment delivered from August 18, 2022, to August 24, 2022. Gross tumor volume (GTV) was contoured on MRI images and checked with the fused contrast-enhanced CT planning images using deep inspiration breath hold. Clinical target volume (CTV) was created using the triangle volumes delineated by Hill et al.1 The GTV dose was prescribed to a dose of 50 Gy in 5 fractions using a daily adaptive technique with a CTV dose of 30 Gy. Treatment was delivered over consecutive days on the MRIdian (ViewRay Inc.) MRI linear accelerator and an intensity-modulated radiation therapy. The daily max and mean doses respectively to the duodenum for each day were 7.11 Gy and 2.75 Gy for day 1, 7.45 Gy and 2.97 Gy for day 2, 6.68 Gy and 2.69 Gy for day 3, 7.13 Gy and 2.70 Gy for day 4, and 7.47 Gy and 2.67 Gy for day 5. The maximum duodenum dose for the entire treatment was 35.84 Gy. A follow-up at 5 and 12 weeks showed normal CA 19-9 of 18.1 and 63.5, and no progression on imaging, and she started surveillance. At 7 months post treatment, she was feeling well and had no symptoms related to her cancer. Her pancreas protocol CT scan, however, revealed migration of the biliary stent. Post-treatment scans and endoscopy showed stent migration and fistula formation between the intrapancreatic distal common bile duct and the duodenum lumen. Conservative management was pursued as she was asymptomatic with adequate biliary decompression and remained symptom free for 11 months thereafter.
Imaging Findings
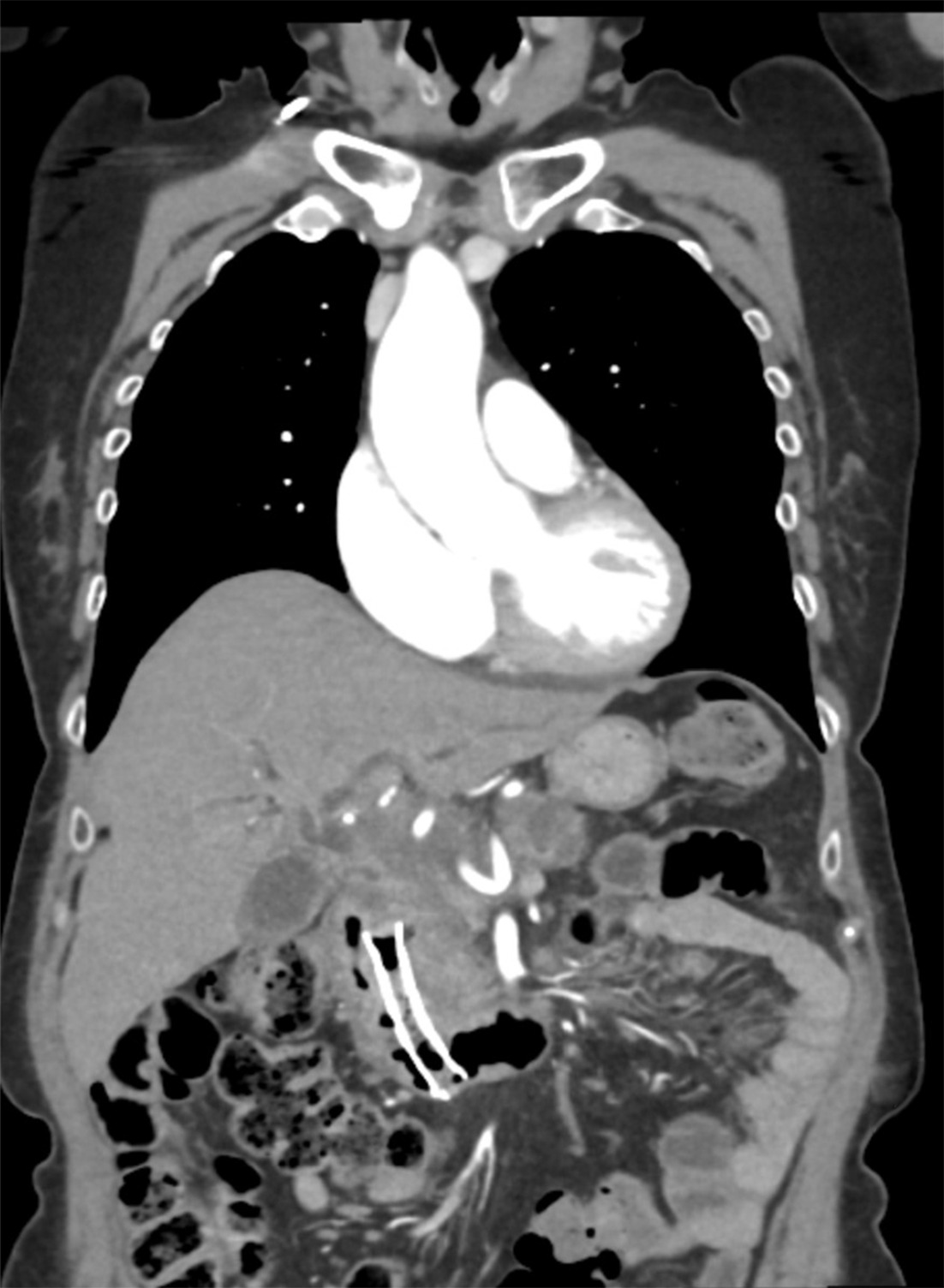
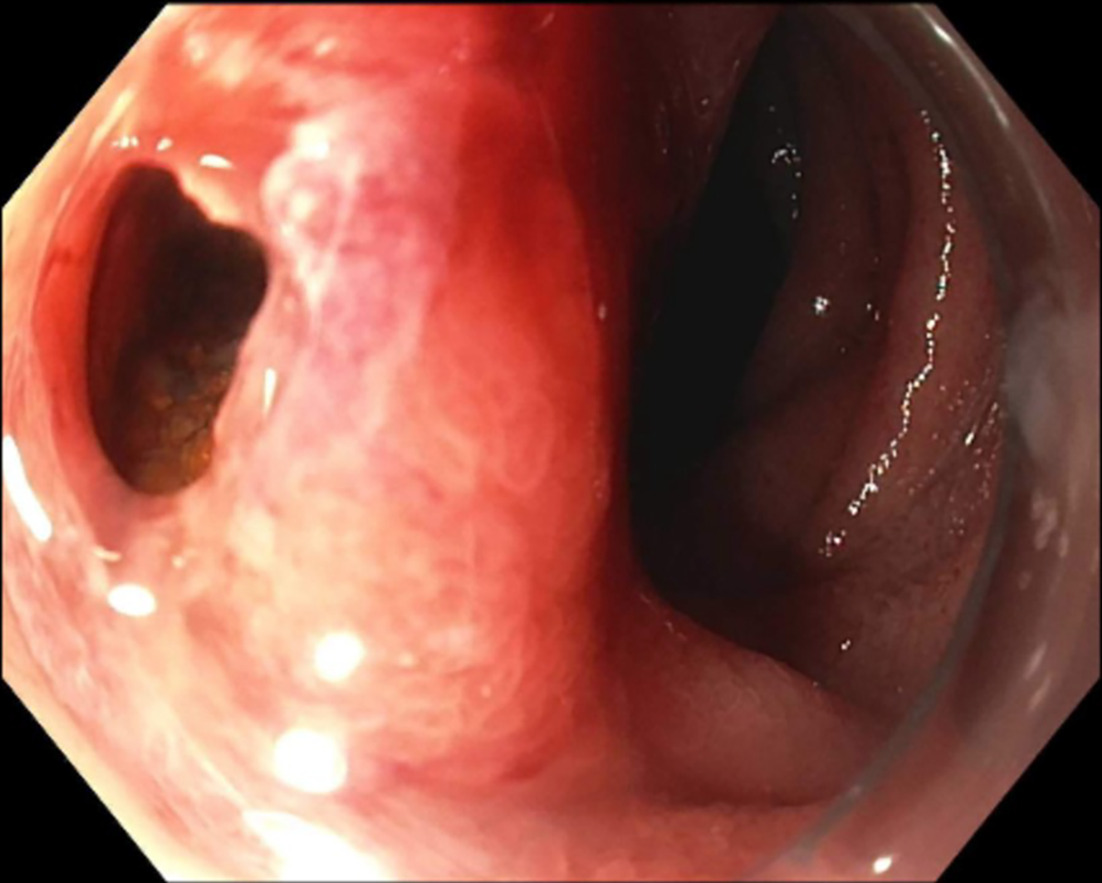
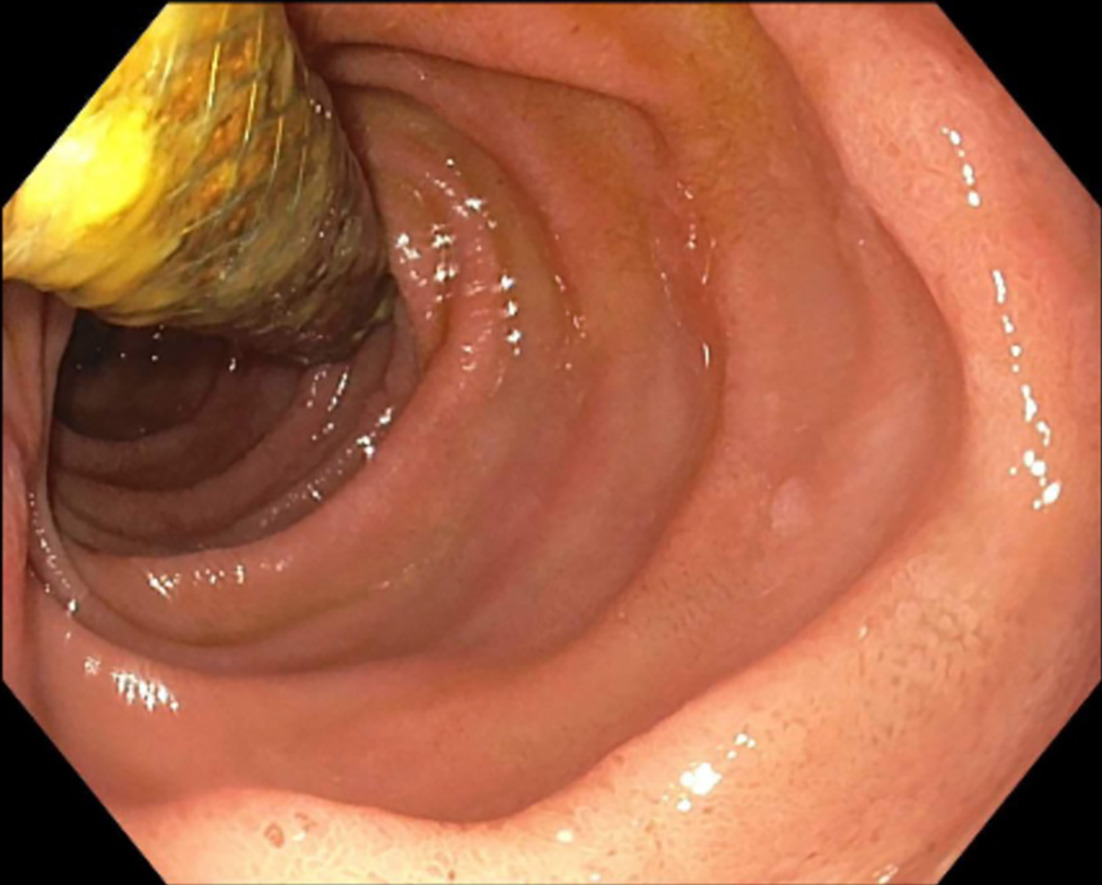
The ERCP and EUS findings helped confirm a poorly defined irregular pancreas head adenocarcinoma with double-duct sign measuring 3.1 × 5.6 × 3.2 cm confirmed through the pancreas protocol CT scan. The tumor encased the portal vein and superior mesenteric vein by more than 180° and was thus staged as locally advanced. Abnormal peripancreatic lymphadenopathy was noted. Routine surveillance pancreas protocol CT scan 7 months post SMART completion unexpectedly showed the biliary stent dislodged within the duodenum ( Figure 1 ). Endoscopic retrieval was attempted but aborted due to a fistulous opening in the duodenum ( Figure 2 ). The proximal aspect of the stent was visualized through the fistulous opening ( Figure 3 ).
Coronal CT image of the migrated stent into the duodenum.
Fistulous opening above the major papilla.
Endoscopic appearance of stent protruding from the fistulous opening proximal to the major papilla.
Diagnosis
At diagnosis, the tumor stage was T4N2M0 and deemed unresectable. The patient’s fully covered metal stent made of metal alloy tubular mesh with a synthetic covering was placed 1 month prior to chemotherapy and 7 months prior to SMART.
Gastrointestinal (GI) endoscopy revealed a 10-mm duodenal fistulous opening proximal to the major papilla. The patient’s biliary stent was inside the fistula opening and had migrated outward. The stent was not removed in the setting of the contained fistula, and the GI endoscopy team recommended conservative management at the time ( Figure 3 ).
A review of the patient’s outside endoscopic procedures prior to coming to our center for cancer treatment showed that the initial ERCP had not been successful. The record indicated that the major papilla appeared bulbous and bulging. The papillary orifice could not be visualized, and attempts to achieve biliary cannulation using a conventional technique via the ampulla of Vater were unsuccessful. A precut technique was then successfully employed.
There was mild bleeding in the second portion of the duodenum, which was managed with epinephrine. The procedure was aborted and a percutaneous transhepatic cholangiogram was done instead to place an internal/external stent. Two days later, she had her internal/external drain removed and a fully covered metal stent placed. At the time of the procedure, a significant hemobilia and blood clots in the bile duct were noted. There was bleeding from the site of the previous papillotomy. A single clip was deployed along the site to successfully stop the bleeding.
Discussion
Locally advanced pancreatic cancer (LAPC) accounts for 30% of newly diagnosed pancreatic cancer, with approximately one-third of the cases becoming resectable after systemic chemotherapy and/or radiation therapy.2 Since most of these tumors remain unresectable, survival rates following systemic chemotherapy and traditional radiation therapy have been low.3 Recent studies of systemic therapy, followed by higher biologically effective dose regimens, either alone4 or with a tumor modifier,5 have reported improved outcomes with low toxicity rates.
In this case, the patient was treated with MRI-guided stereotactic body radiation therapy to a dose of 50 Gy in 5 fractions following 6 months of systemic FOLFIRINOX using daily adaptation. The precision provided by SMART allows for both higher control rates and lower toxicity rates. A recent prospective phase 2 study of both borderline patients and patients with LAPC reported 2-year rates of local control of 78.2% and overall survival of 40.5% following treatment with no acute-grade ≥3 acute toxicity and minimal late-grade ≥ 3 toxicity.4 While acute-, late-, and high-grade toxicities are minimized using SMART, they can still occur.
The patient had a complicated course following her treatment with chemotherapy and radiation, including stent migration and fistula formation. The location of the fistula was in the precise region where the needle-knife fistulotomy was performed. The antecedent injury to the biliary mucosa was associated with bleeding during and following the procedure, so much so that an endoscopic clip had to be placed to stop the bleeding. Although we cannot be certain as to the exact etiology of the post-treatment-related fistula, the iatrogenic mucosal defect may have been a predisposing factor in this case. The importance of multidisciplinary evaluation with gastroenterology to individualize risk assessments for each case is important. Specifically, pretreatment endoscopy prior to radiation therapy can provide critical information to the radiation oncologist when tailoring the treatment plan. In this case, the patient is now 18 months post treatment and remains asymptomatic. No endoscopic intervention has been required to date since the identification of the duodenal fistula.
Grade 2 and above rates of GI toxicity postablative radiation therapy have been seen at rates of 2% to 47%, including biliary tract injuries of ulcers, fistulas, and gastric or duodenal mucositis.2, 3, 6, 7 Grade 3-4 events such as ulcers, fistulas, and gastric or duodenal mucositis have been seen at rates of 13% to 22.7%.3 For duodenal and other GI fistulas, depending on the severity and containment of the fistula, treatments can vary from conservative management, advanced surgical procedures, and varying endoscopic techniques.8, 9 In patients with endobiliary stents, there are reported complications associated with radiation therapy. Minimal stent position changes have been described following RT, with stent migration rates of 4.9% proximally and 5.9% distally.10 However, adverse events such as stent migration and fistula formation occur more frequently in patients who have undergone previous chemoradiotherapy treatment vs those who have undergone stent placement alone.11 The significant interfraction deformation of GI structures, such as the pancreatic head, duodenum, and stomach that may occur during radiation treatment, may affect stent migration.10 Additionally, a dilated common bile duct, complete sphincterotomy, prolonged stent insertion, benign strictures, and covered metal stents were the factors associated with stent migration.12, 13
Conclusion
This case highlights the potential of significant adverse events despite the evolution of advanced techniques such as SMART. This patient was considered lower risk for adverse events such as stent migration or fistula formation due to her history of no chemoradiation prior to diagnosis. Nonetheless, a fistula still occurrs even with the precision and lower toxicity rates of a modern radiation therapy technique. Both gastroenterologists and radiation oncologists should be aware of the potential predisposing factors for fistula formation. In this case, an iatrogenic factor (attempted needle-knife fistulotomy) likely predisposed the patient to develop a fistula post radiation. In the future, knowledge of predisposing endoscopic factors may guide radiation oncologists in modifying from a 5-fraction to a 25-fraction dose plan.14
References
Citation
Sarah Goodchild; Ann Nguyen, BS; Russell Palm, MD; Brian Morse, MD; Dae Won Kim, MD; Pam Hodul, MD; J. M. Bryant, MD; Sarah Hoffe, MD; Aamir Dam, MD; Jessica Frakes, MD. Stent Migration and Fistula Formation Following Stereotactic Body Radiation Therapy for Pancreatic Cancer. Appl Radiat Oncol. 2024;(4):1 - 4.
doi:10.37549/ARO-D-24-00008
December 1, 2024