SBRT vs SABR: Does Terminology Differentiate Treatment Intent in Metastatic Cancer?
Images

SA-CME credits are available for this article here.
Stereotactic body radiation therapy (SBRT) or stereotactic ablative radiation therapy (SABR) has an evolving role in the treatment of primary and metastatic cancer. Loosely defined in the United States as radiation therapy that delivers high-dose radiation within a single or very few (generally ≤ 5) fractions, various terms have been used interchangeably to describe stereotactic radiation therapies with no clear-cut terminology documented.1 The term SABR emerged in 2010 as it was thought to more accurately describe the dose intensity of the treatment vs SBRT, and it was proposed that the term be used instead of SBRT.2 Several trials on stereotactic radiation therapies have since been developed and published, yet the discourse surrounding preferred terminology within the literature remains unclarified.
Previous work has shown that patients undergoing treatment for metastatic cancer often do not have an accurate understanding of the intent of therapy, and that this misunderstanding may influence their decisions about further treatments.3-5 In the setting of metastatic disease, stereotactic therapies may be administered with intent to either ablate disease or provide palliation. Confusion results from the interchangeable use of the term SABR, which implies ablative intent, and SBRT, which is agnostic toward treatment intent. We anticipate that clarification of this terminology could help avoid confusion for patients and physicians, ultimately improving communication with patients undergoing treatment for metastatic disease. Herein, we review published prospective trials and protocols on stereotactic radiation therapies for metastatic disease to determine whether the terms SBRT and SABR are currently being used differentially based on intent of treatment, defined by primary study outcome, and propose a distinct definition of each.
Evidence Review
We conducted a narrative review of the literature to identify and summarize prospective trials and protocols that investigated the use of stereotactic radiation therapies for patients with metastatic disease. A PubMed query was conducted (search query outlined in Supplementary Text 1 available with the online version of this article at www.appliedradiationoncology.com). Trials and protocols were included if: 1) they evaluated the use of radiation therapy directed toward visceral or bone metastases, 2) the intervention included stereotactic radiation therapy, they assessed a primary outcome related to treatment response, disease control, or quality of life, 4) they used a prospective study design, and 5) they were published between January 1, 2010 and September 5, 2020. Studies were excluded if 1) they were phase 1 or pilot studies, they represented a secondary analysis of a previously published trial, or 3) they included pediatric patients. A hand search of the gray literature included relevant professional organization websites as well as ClincialTrials.gov.
We categorized the trials we identified based on terminology used (SBRT vs SABR), and whether they were single arm or randomized. We also categorized the primary endpoint in each study based on treatment intent, either as “tumor control” if related to local control, progression-free survival, or overall survival; or “palliation” if related to relief of symptoms. Inclusion and categorization of each study was determined by 2 reviewers (KL, NJM), and discrepancies were resolved by a third reviewer (EFG). Fisher’s exact test was used to assess the association between trial terminology and primary endpoint category.
Findings
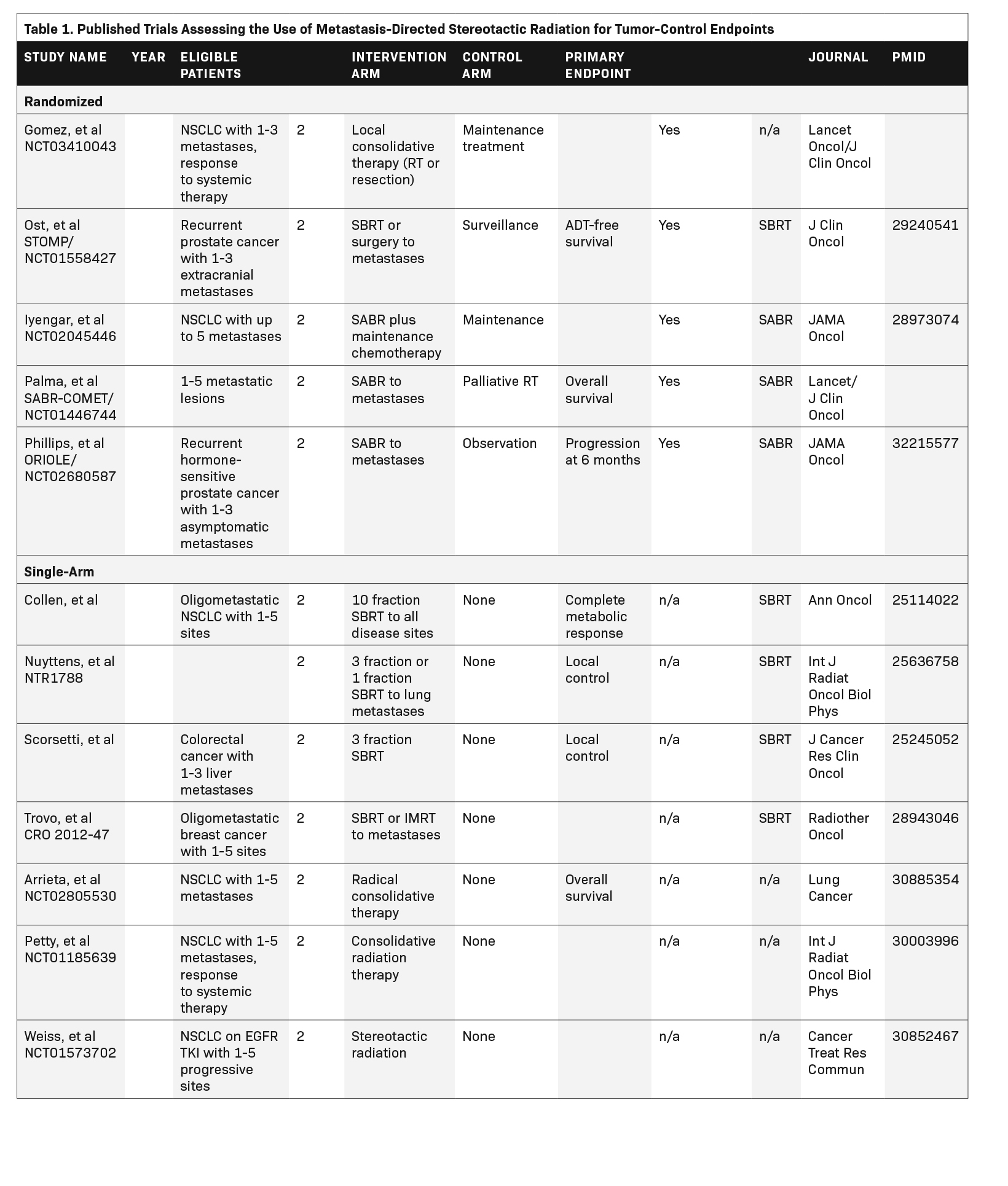
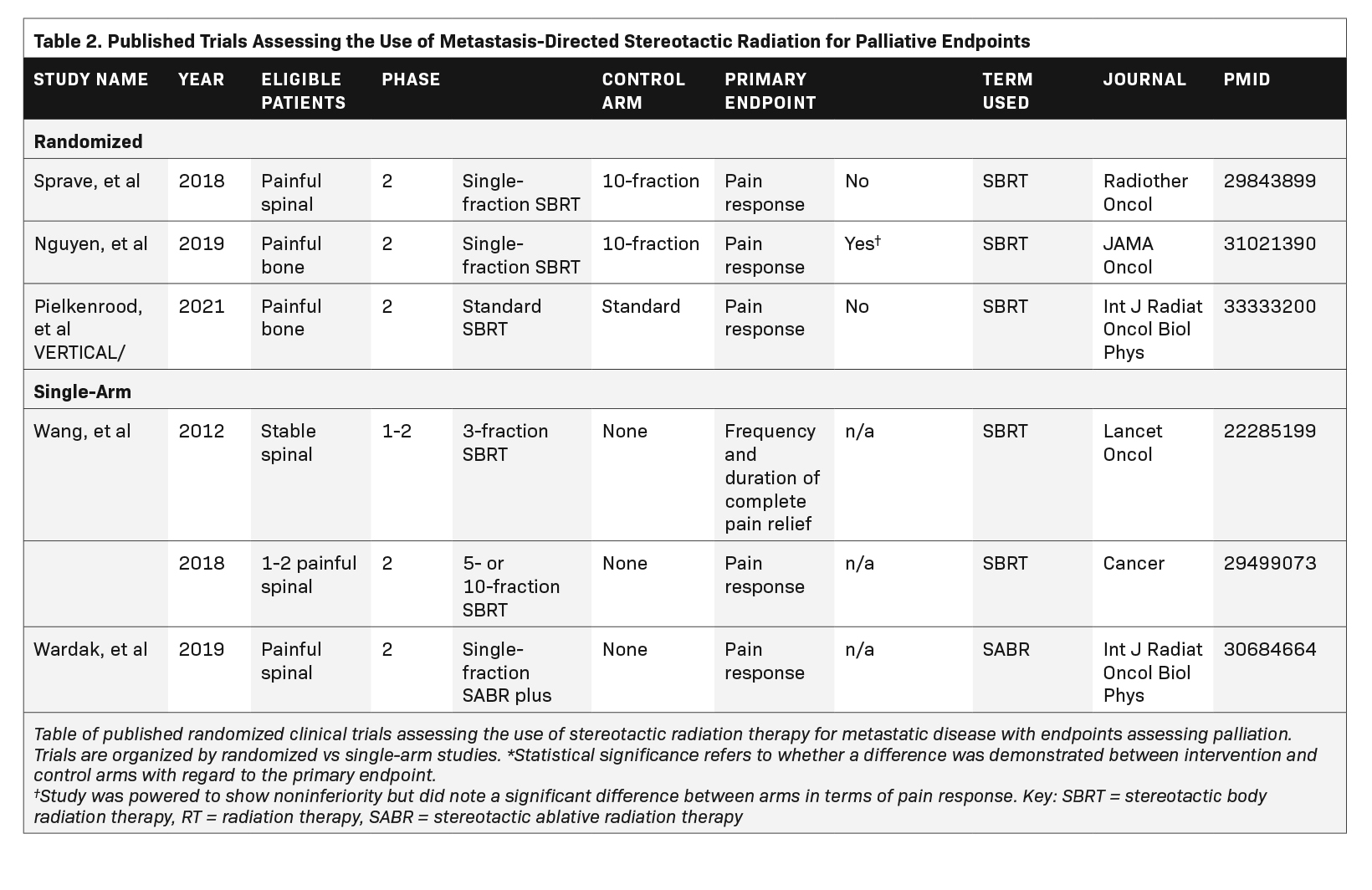
Overall, 48 trials met eligibility criteria, of which 40% (n = 19) had published their results,6-25 and 60% (n = 29) were ongoing. Published studies are listed in Table 1 and Table 2. Eight trials (17%) primarily used the term SABR, 36 (75%) used the term SBRT, and 4 (8%) used a different term to describe the intervention. Nineteen trials (40%) were randomized. Overall, 75% (n = 36) and 25% (n = 12) of the trials assessed a primary outcome categorized as tumor control or palliation, respectively. Primary outcome did not differ based on intervention terminology (P = 0.41). We also assessed the use of terminology in published randomized trials only, speculating that these are usually the most influential publications. This subset includes 8 studies, of which 4 use the term SBRT, 3 use the term SABR, and 1 uses local consolidative therapy. Of note, within this cohort, all studies assessing palliative endpoints used the term SBRT, and 3 of 5 studies assessing tumor control used the term SABR.
Discussion
Recent evidence from randomized clinical trials has shifted the way in which we approach the treatment of patients with limited metastatic disease, expanding indications for the use of stereotactic radiation therapy with curative intent.26 An updated analysis of the phase 2 SABR- COMET (NCT01446744) trial reported a median 22-month increase in overall survival at 5 years with SABR in patients with controlled primary yet oligometastatic disease compared to standard of care.11 Other randomized trials that have investigated the effect of stereotactic therapies on outcomes related to survival and disease progression include Gomez et al (NCT01725165),6,7 Iyengar et al (NCT02045446),9 and the ORIOLE trial (NCT02680587).12 The trials published by Gomez et al and Iyengar et al are both phase 2 randomized trials that showed prolonged progression-free survival in patients with oligometastatic non-small-cell lung cancer who received SABR compared with maintenance therapy. The recently published ORIOLE trial showed lower rates of disease progression at 6 months in patients with oligometastatic prostate cancer who received SABR compared with observation. Collectively, these trials suggest that SABR/SBRT may effectively prolong progression-free and overall survival in patients with oligometastatic disease, typically defined as disease with limited metastases to 1 or 2 other regions of the body outside of the site of primary disease.27
While stereotactic radiation therapy has a promising role in the curative treatment of patients with oligometastatic disease, it also has an emerging role in the palliation of symptoms caused by metastatic cancer. Prospective studies have shown that SBRT is feasible in the palliation of bone metastases and may reduce cost and the amount of time patients spend receiving treatment.28,29 A recent randomized trial conducted at The University of Texas MD Anderson Cancer Center (NCT02163226) found that the use of SBRT vs standard multifraction radiation therapy for the treatment of symptomatic bone metastases resulted in higher rates of pain response.21 An additional phase 2 randomized trial published by Sprave et al (NCT02358720) found a more rapid and durable pain response with SBRT compared with multifraction conventional palliative radiation therapy (30 Gy in 10 fractions) for patients with spinal bone metastases.20 Although further evidence is needed before the efficacy of SBRT for the palliative treatment of bone metastases is fully understood and recommended for use in routine practice, there are technical advantages to this modality, and its use in the palliative setting has been increasing.30,31
Despite the proposal to switch from the term SBRT to SABR in 2010,2 several trials still use the term SBRT. The results of our literature review demonstrate no correlation between terminology and treatment intent amongst all studies, but when including only the most influential publications (randomized trials), there seems to be a selective choice in terminology based on the endpoint. Given these findings, it may be reasonable to suggest that the term SABR should refer to a type of stereotactic therapy that is delivered with curative intent for patients with metastatic disease, and that it is not synonymous but rather falls under the more encompassing term SBRT in this setting. We recognize that for most clinicians, the terms SBRT and SABR are often considered interchangeable, despite prior calls to standardize terminology. The term SABR represents a newer name for an already existing treatment and is thought to more accurately describe the dose intensity in addition to its aesthetic benefits. Nonetheless, the interchangeable use of the terms in clinical practice, despite the preference for the term SABR when publishing randomized trials aimed at tumor control for metastatic disease, likely creates unnecessary confusion.
Conclusion
Evidence is evolving on the use of stereotactic radiation therapies for both palliative and ablative treatment in the metastatic disease arena. With this split in the paradigm, there is an important opportunity to improve clarity surrounding treatment intent by using consistent terminology. Based on our review of published randomized control trials and protocols, the term SABR is more commonly used in the literature for oligometastatic disease in which stereotactic radiation therapy is administered with curative intent. In contrast, the term SBRT is more widely used and encompasses radiation therapy delivered with both palliative and curative intent to patients with incurable metastatic disease and oligometastatic disease, respectively. Therefore, we propose that a distinction be made and that the term SABR should be used in reference to stereotactic therapies delivered with curative intent for patients with oligometastatic disease, while the term SBRT should be used to describe radiation therapy delivered with palliative intent to sites of metastases regardless of overall disease burden. We believe this distinction will reduce confusion in routine practice and ensure consistency in the publication of research on a single technique used for two distinct purposes.
References
- Tipton K, Launders JH, Inamdar R, Miyamoto C, Schoelles K. Stereotactic body radiation therapy: scope of the literature. Ann Intern Med. 2011;154(11):737-745.
- Loo BW, Jr., Chang JY, Dawson LA, et al. Stereotactic ablative radiotherapy: What’s in a name? Pract Radiat Oncol. 2011;1(1):38-39.
- Matsuyama R, Reddy S, Smith TJ. Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol. 2006;24(21):3490-3496.
- Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616-1625.
- Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279(21):1709-1714.
- Gomez DR, Blumenschein GR, Jr., Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672-1682.
- Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558-1565.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
- Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non- small-cell lung cancer. J Clin Oncol. 2014;32(34):3824-3830.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051-2058.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR- COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830-2838.
- Phillips R, Shi WY, Deek M, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6(5):650-659.
- Collen C, Christian N, Schallier D, et al. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol. 2014;25(10):1954-1959.
- Nuyttens JJ, van der Voort van Zyp NC, Verhoef C, et al. Stereotactic body radiation therapy for oligometastases to the lung: a phase 2 study. Int J Radiat Oncol Biol Phys. 2015;91(2):337-343.
- Trovo M, Furlan C, Polesel J, et al. Radical radiation therapy for oligometastatic breast cancer: results of a prospective phase II trial. Radiother Oncol. 2018;126(1):177-180.
- Arrieta O, Barron F, Maldonado F, et al. Radical consolidative treatment provides a clinical benefit and long-term survival in patients with synchronous oligometastatic non-small cell lung cancer: a phase II study. Lung Cancer. 2019;130:67-75.
- Petty WJ, Urbanic JJ, Ahmed T, et al. Long-term outcomes of a phase 2 trial of chemotherapy with consolidative radiation therapy for oligometastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2018;102(3):527-535.
- Weiss J, Kavanagh B, Deal A, et al. Phase II study of stereotactic radiosurgery for the treatment of patients with oligoprogression on erlotinib. Cancer Treat Res Commun. 2019;19:100126.
- Redmond KJ, Sciubba D, Khan M, et al. A phase 2 study of post-operative stereotactic body radiation therapy (SBRT) for solid tumor spine metastases. Int J Radiat Oncol Biol Phys. 2020;106(2):261-268.
- Sprave T, Verma V, Forster R, et al. Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three- dimensional conformal radiotherapy. Radiother Oncol. 2018;128(2):274-282.
- Nguyen QN, Chun SG, Chow E, et al. Single-fraction stereotactic vs conventional multifraction radiotherapy for pain relief in patients with predominantly nonspine bone metastases: a randomized phase 2 trial. JAMA Oncol. 2019;5(6):872-878.
- Pielkenrood BJ, van der Velden JM, van der Linden YM, et al. Pain response after stereotactic body radiation therapy versus conventional radiation therapy in patients with bone metastases-a phase 2 randomized controlled trial within a prospective cohort. Int J Radiat Oncol Biol Phys. 2021;110(2):358-367.
- Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol. 2012;13(4):395-402.
- Guckenberger M, Sweeney RA, Hawkins M, et al. Dose-intensified hypofractionated stereotactic body radiation therapy for painful spinal metastases: results of a phase 2 study. Cancer. 2018;124(9):2001-2009.
- Wardak Z, Bland R, Ahn C, et al. A phase 2 clinical trial of SABR followed by immediate vertebroplasty for spine metastases. Int J Radiat Oncol Biol Phys. 2019;104(1):83-89.
- Onderdonk BE, Gutiontov SI, Chmura SJ. The evolution (and future) of stereotactic body radiotherapy in the treatment of oligometastatic disease. Hematol Oncol Clin North Am. 2020;34(1):307-320.
- NCI Dictionaries. In: NIH National Cancer Institute. Accessed June 1, 2021. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/oligometastasis
- Muller DA, Wages NA, Wilson DD, et al. STAT RAD: prospective dose escalation clinical trial of single fraction scan-plan-qa-treat stereotactic body radiation therapy for painful osseous metastases. Pract Radiat Oncol. 2020;10(6):e444-e451.
- Wilson DD, Alonso CE, Sim AJ, et al. STAT RT: a prospective pilot clinical trial of Scan-Plan-QA-Treat stereotactic body radiation therapy for painful osseous metastases. Ann Palliat Med. 2019;8(3):221-230.
- Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: update of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7(1):4-12.
- Gharzai LA, Beeler WH, Hayman JA, et al. Recommendations for single-fraction radiation therapy and stereotactic body radiation therapy in palliative treatment of bone metastases: a statewide practice patterns survey. Pract Radiat Oncol. 2019;9(6):e541-e548.
Citation
K L, NJ M, CJ T, JT Y, EF G. SBRT vs SABR: Does Terminology Differentiate Treatment Intent in Metastatic Cancer?. Appl Radiat Oncol. 2021;(4):6-10.
December 28, 2021