Review ArticleGenitourinary Cancer
SBRT With Integrated Boost to the Dominant Intraprostatic Nodule: Initial Dosimetric and Clinical Outcomes
Images

Abstract
Purpose: Prostate stereotactic body radiation therapy (SBRT) with dose escalation to the dominant intraprostatic lesion (DIL) is an option to increase local control. We investigated the feasibility and toxicity of a moderate boost to DIL while respecting established dose constraints.
Materials and Methods: Ten patients with prostate cancer who met eligibility criteria for NRG-GU005 were included. T2-weighted MRI and planning pelvis computed tomography (CT) were fused to delineate targets and organs at risk (OARs). SBRT plans (36.25 Gy in 5 fractions) followed GU005 constraints. Paired T-tests were used for analysis of dosimetrics. Early (< 90 days) and late (> 90 days) genitourinary (GU) and gastrointestinal (GI) toxicity were graded by National Cancer Institute Common Terminology Criteria.
Results: Mean prescription dose (36.25 Gy) coverage of the planning target volume (PTV) was 95.4%. The conformity index for all plans was < 1.2, and the dose to 0.03 cc of the PTV was < 120% of the prescription dose (43.50 Gy). The minimum dose (D99%) of the PTV was 35.1 ± 0.4 Gy, whereas the D99% for DIL was 38.6 ± 0.8 Gy. Using an alpha/beta of 1.5, BED was 199.4 Gy for PTV vs. 237.3 Gy for DIL, P < 0.001. The incidence of acute grade 1 or 2 GI toxicity was 20%, of which 10% persisted past 90 days. The incidence of acute GU toxicity was 80%, of which 50% persisted past 90 days. No patients developed grade 3 or greater GI or GU toxicity.
Conclusion: Prostate SBRT with simultaneous moderate dose escalation to DIL is feasible and can be accomplished while respecting standard OAR constraints.
Prostate adenocarcinoma is the noncutaneous cancer with the highest incidence among men in the US; there were 174,650 new prostate cancer cases in the US in 2019.1 According to the Surveillance, Epidemiology, and End Results (SEER) program, 77% of new prostate cancer cases in 2019 were diagnosed as localized stage.2
Based on National Comprehensive Cancer Network (NCCN) guidelines, treatment options for low- to intermediate-risk prostate cancer include active surveillance, surgery, and radiation therapy.3 Radiation is delivered either via external-beam radiation therapy (EBRT) or brachytherapy (BT), with the potential addition of androgen deprivation therapy for unfavorable intermediate-risk groups. Previous studies have shown that dose escalation in EBRT is associated with improved biochemical control and progression-free survival but is not associated with improved overall survival.4-7 With dose-escalated EBRT, care must be taken in radiation treatment planning, as there can be greater risk of toxicity from increased dose to organs at risk (OARs), including the rectum and bladder.8
Prior research has shown that prostate cancer is characterized by a low alpha/beta ratio of approximately 1.5 Gy.9-11 This gives rise to a potential benefit of hypofractionated radiation therapy, where radiation is delivered in higher daily doses over fewer total fractions, in terms of achieving greater tumor control while minimizing toxicity. Stereotactic body radiation therapy (SBRT) is a form of extreme hypofractionation; one common regimen used in prostate cancer is 36.25 Gy in 5 fractions (7.25 Gy per fraction), as per the NRG RTOG-0938 trial.12 Studies suggest that SBRT for treatment of low- to intermediate-risk prostate cancer is associated with improved biochemical relapse-free survival, with an acceptable toxicity profile.13-15
Studies of patterns of failure in EBRT with standard fractionation show the area of local recurrence is the dominant intraprostatic lesion (DIL) in approximately 90% to 100% of cases.16,17 Therefore, the DIL is a favorable target for heterogeneous dose escalation in SBRT. A phase I trial conducted by Herrera et al demonstrated promising anti-tumor activity and minimal toxicities associated with SBRT and simultaneous dose escalation to the DIL up to 50 Gy in 5 fractions.18
The objective of this study is to investigate the efficacy and toxicity profile of a moderate boost to the DIL while following NRG-GU005 dose constraints. We hypothesized that it is feasible to boost the DIL during SBRT and that doing so while following established dosimetric constraints would result in a favorable toxicity profile.
Materials and Methods
Subjects
We retrospectively reviewed 10 consecutive patients treated at our institution between October 2017 and December 2018 with definitive SBRT (36.25 Gy in 5 fractions) for intermediate-risk prostate cancer; these patients were treated with SBRT following the dose contouring guidelines and dose constraints from the SBRT arm of NRG-GU005 to guide treatment planning. No patients received hormone therapy. We retrospectively analyzed clinical characteristics, dosimetry, and toxicity of these patients by chart review. This study was approved as exempt by the institutional review board (IRB).
Treatment Planning
All patients had gold fiducial markers (Civco Medical Solutions) placed in the prostate prior to treatment. Four fiducial markers were placed, and they were placed at least 1 cm apart, when possible. For SBRT planning, both a thin slice (1 to 1.5 mm) noncontrast pelvic CT and a high-resolution nonendorectal coil 1.5 T or 3 T MRI were used. Axial T2-weighted turbo spin echo images provided anatomical information, and axial noncontrast T1-weighted gradient-echo images were used for fiducial marker localization, both in the same straight axial orientation as CT slices with slice thickness of 2 to 3 mm. To accurately identify the DIL, MRI THRIVE images (dynamic contrast-enhanced gradient-echo sequences) were also acquired and fused with planning CT.
Specific preparation instructions were given to all patients to minimize prostate motion during SBRT simulation and treatment. Two days prior to CT simulation, patients were advised to follow a low-gas, low-motility diet. The day prior to simulation, patients were instructed to use a mild laxative and a gas relief medication, as well as to change their diet to a clear liquid diet. On the day of the scan, they were instructed to take a gas relief medication 2 hours prior to their appointment time. Patients were also instructed to empty their bladders before scans. All patients were imaged and treated in supine positions with a SBRT body frame (Bionix). A knee cushion was used when necessary for patient comfort. None of the patients had a rectal spacer, such as SpaceOAR, placed. CT and MRI images were fused in MIMVista (MIM Systems).
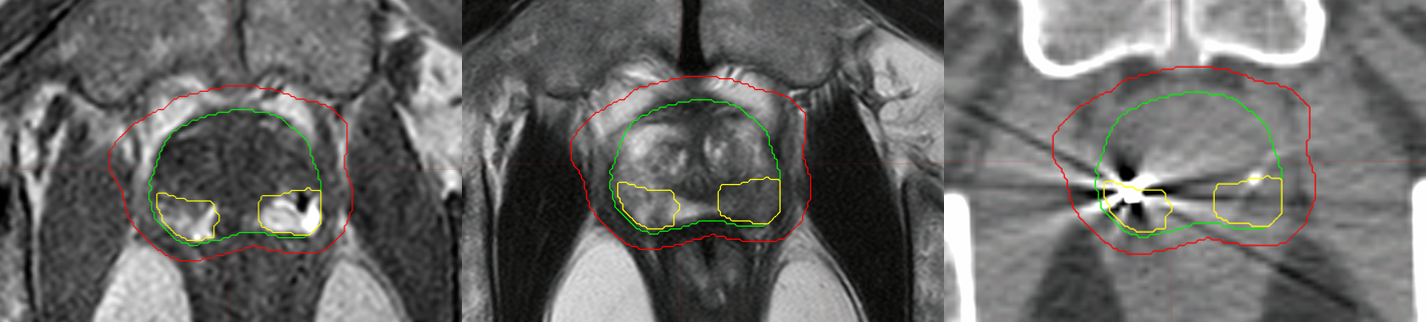
Per NRG-GU005 protocol, the gross tumor volume (GTV) was the prostate only, as defined on T2-weighted MRI. The clinical target volume (CTV) was the prostate and approximately 1 cm of proximal seminal vesicles, and the planning target volume (PTV) was 5-mm expansion in all directions, except 3 mm posteriorly on the CTV. The DIL and urethras were contoured with the help of a genitourinary radiologist using THRIVE and T2-weighted MRIs (Figure 1).
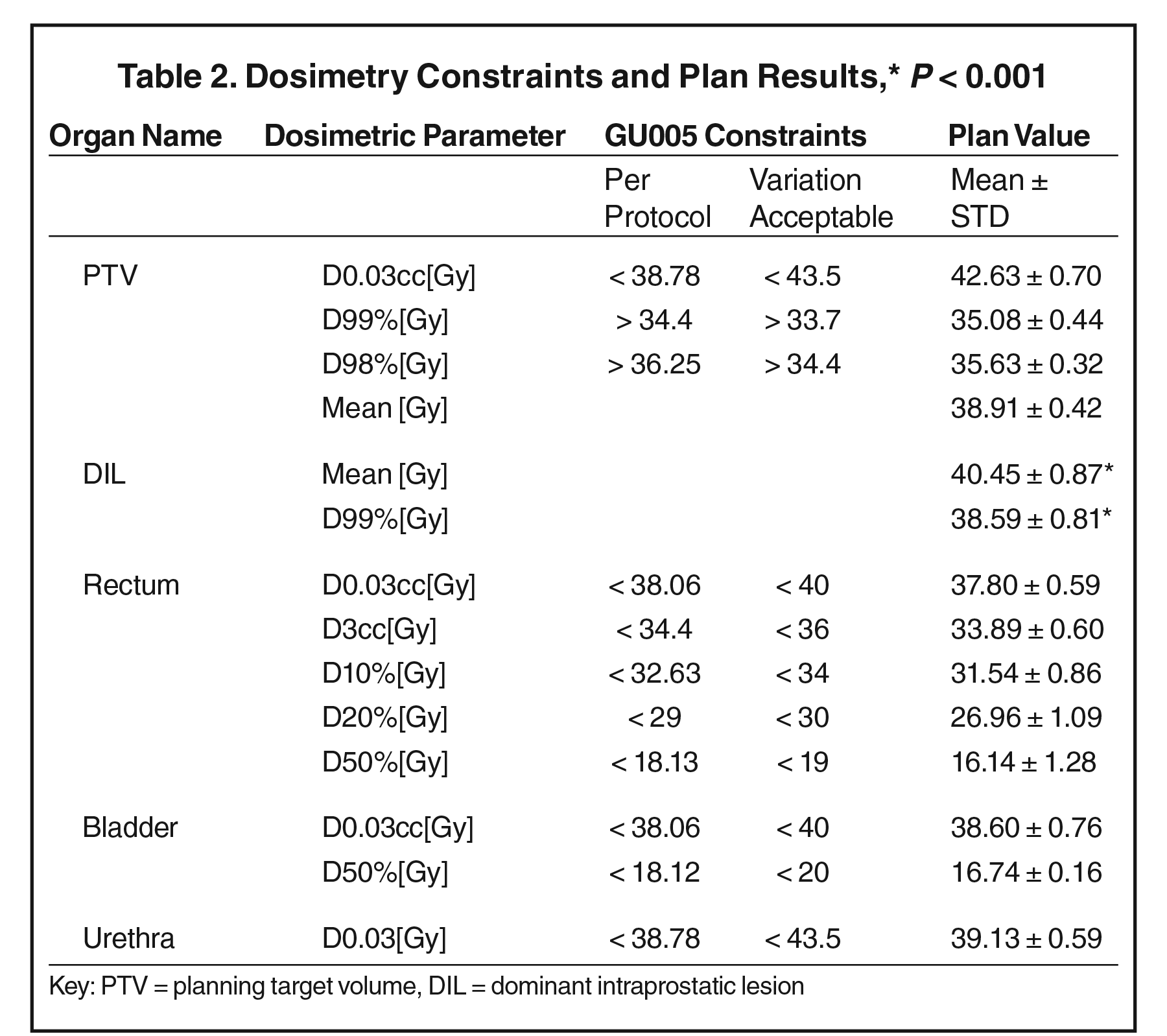
Treatment plans (36.25 Gy in 5 fractions) were generated with MultiPlan 4.6.1 using sequential optimization. Hot spots (< 120% of prescription dose) inside the PTV were intentionally placed in the DIL region by using the objective constraints. Dose-volume histogram (DVH) constraints for the PTV and OARs from protocol NRG-GU005 were followed (Table 2).
SBRT Treatment Delivery
The same bowel regimen used prior to simulation was also used prior to treatment delivery, and all patients were treated on an empty bladder to maximize reproducibility. The entire course was completed within two weeks on an every-other-day basis (eg, M, W, F). SBRT was delivered using the CyberKnife Robotic Radiosurgical System (Accuray Inc.). The CyberKnife system is equipped with two orthogonal kV x-ray imaging devices for image guidance. Fiducial markers can be clearly identified in kV x-ray images attributed to its high density. During treatment delivery, the positions (6D) of fiducial markers were tracked based on the paired kV x-ray images. Imaging was every 15 to 30 seconds to ensure submillimeter tracking accuracy. The robotic system automatically corrected for 6D shifts up to 1-cm translational shifts, 5-degree roll, 2-degree pitch, and 3-degree yaw. If the motion was beyond these limits or noted to be excessive, most commonly due to movement of bowel gas, the treatment was paused until the fiducial orientation/position was back within tolerance.
Follow-up and Toxicity Assessment
Patients were seen in follow-up by the treating radiation oncologist every 3 to 6 months after completion of SBRT. Toxicity and PSA measurements were recorded in medical records as part of standard clinical practice. Retrospectively, genitourinary (GU) and gastrointestinal (GI) toxicity were graded by National Cancer Institute Common Terminology Criteria (NCI-CTC) via chart review. Specific GU symptoms evaluated include dysuria, urinary frequency, urgency, retention, and GI symptoms include proctitis, hemorrhoids, rectal pain, and bleeding. Both early (< 90 days from first fraction) and late (> 90 days from first fraction) toxicities were assessed.
Statistical Analysis
Paired T-tests were used to compare the dose to the entire PTV vs the dose to the DIL.
Results
Subjects
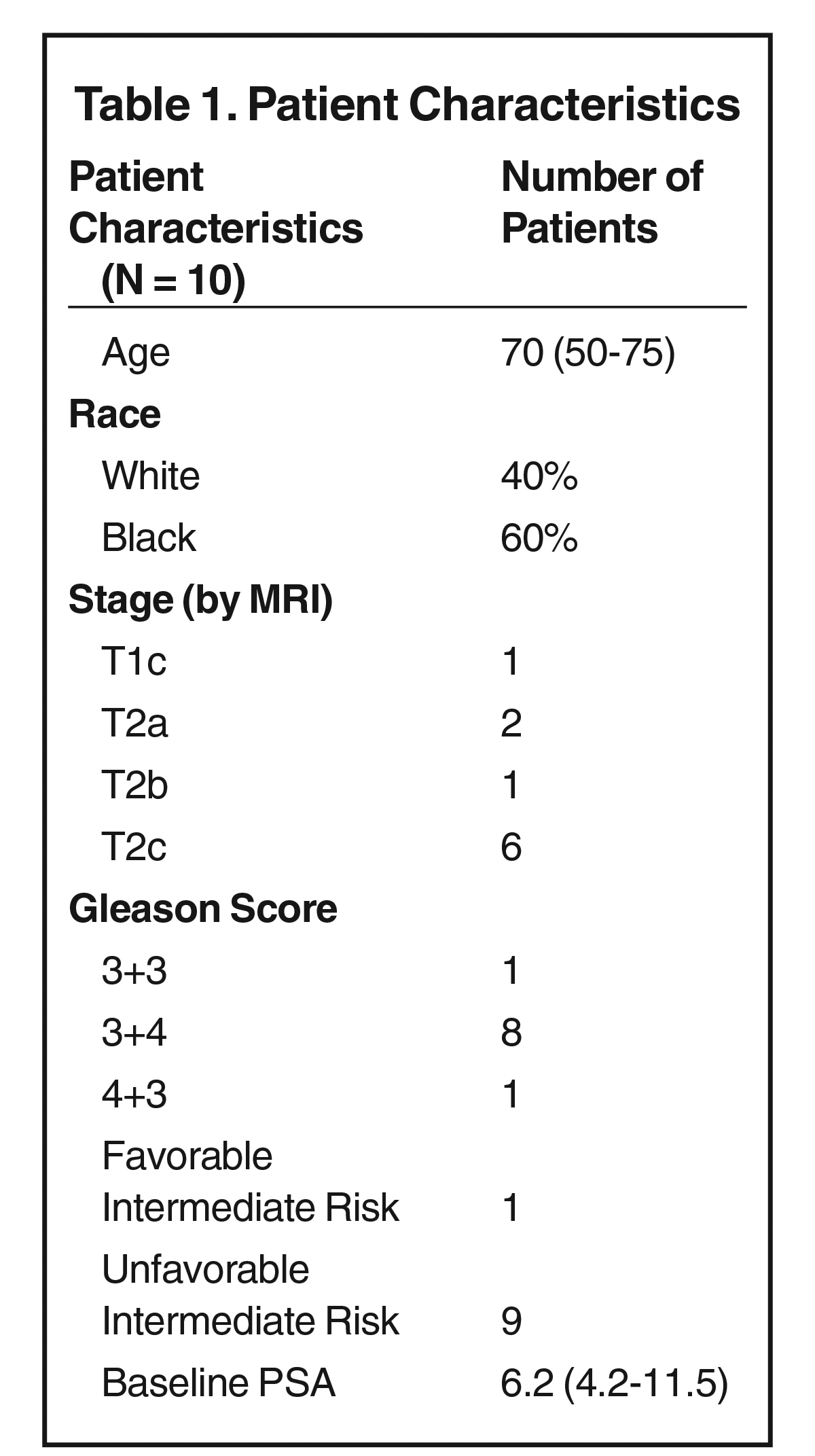
Clinical characteristics are listed in Table 1.
Dosimetry
Target coverage and normal tissue dose constraints are listed in Table 2.
All NRG-GU005 protocol dosimetric constraints were met (Table 2). Mean prostate volume was 39 cc (range 26-59 cc), and mean DIL volume was 2 cc (range 0.7 to 4.5 cc). Mean prescription dose (36.25 Gy) coverage of the PTV was 95.4% (range 93.8 to 97.9%). The conformity index for all plans was < 1.2, and the dose to 0.03 cc of the PTV was 42.63 Gy (range 41.13 to 43.39 Gy), < 120% of the prescription dose (43.50 Gy). The minimum dose received by 99% of the PTV (D99%) was mean 35.1 ± 0.4 Gy, whereas the mean D99% for the DIL was 38.6 ± 0.8 Gy (P < 0.001). Using an alpha/beta of 1.5, this corresponds to a BED of 199.4 Gy for PTV vs. 237.3 Gy for DIL.
For OARs, mean rectum D0.03 cc was 37.8 ± 0.6 Gy, D3cc was 33.89 ± 0.60 Gy, D10% was 31.54 ± 0.86 Gy, and D50% was 16.1 ± 1.3 Gy. Mean bladder D0.03cc was 38.6 ± 0.8 Gy, and mean bladder D50% was 16.7 ± 1.6 Gy (Table 2). D0.03cc to the urethra was mean 39.13 ± 0.59 Gy.
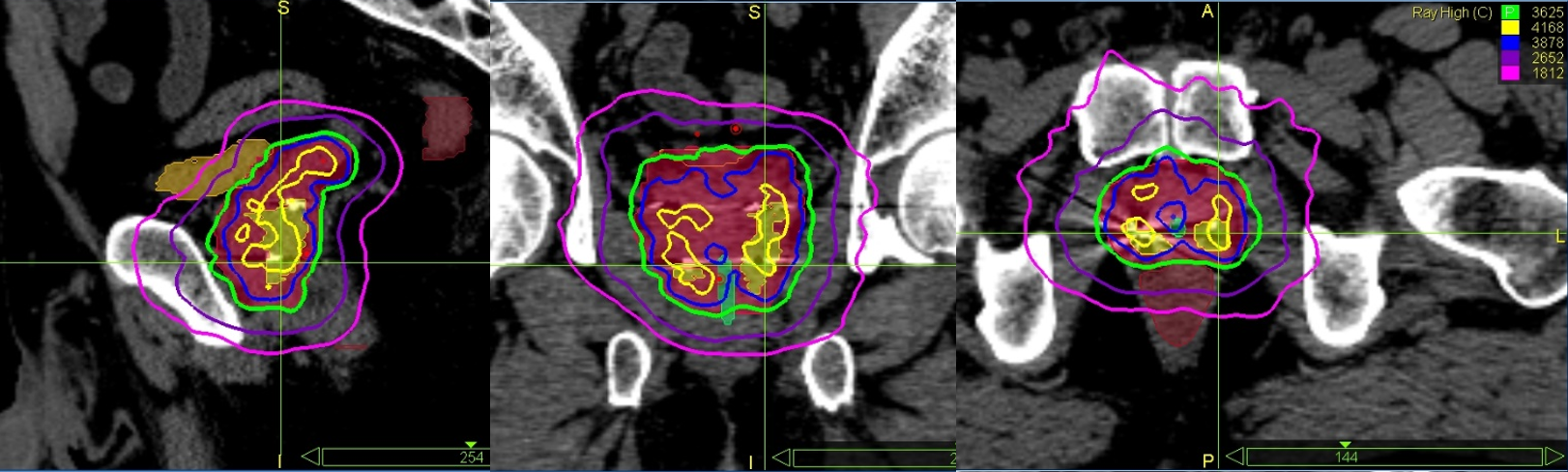
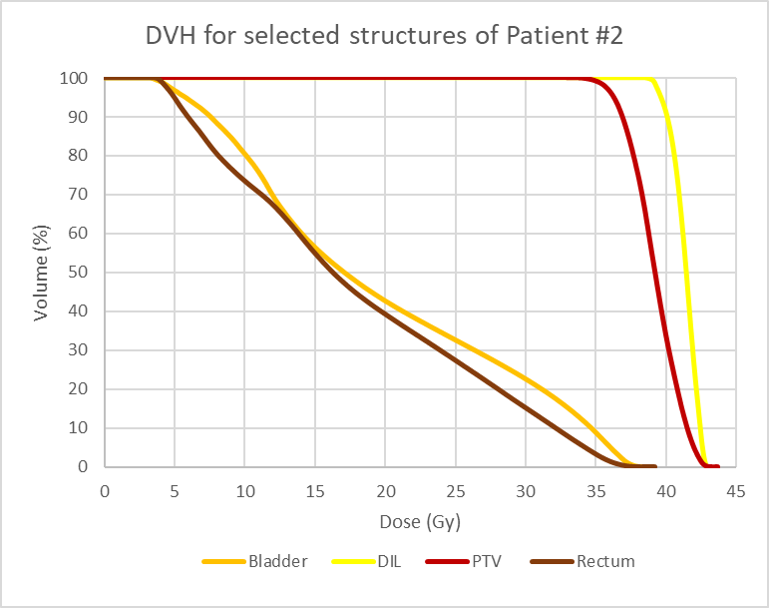
Figure 2 shows screenshots of the graphic plan for patient #2, where higher dose lines (115%) are concentrated in and around the DIL. Figure 3 is the corresponding DVH.
Toxicity
All patients had one on-treatment visit (OTV). The median follow-up time after treatment was 12 months. The incidence of acute grade 1 or 2 GI toxicity was 20%, of which 10% persisted past 90 days. The incidence of acute GU grade 1 or 2 toxicity was 80%, of which 50% persisted past 90 days. No patients developed grade 3 or greater GI or GU toxicity (Table 3).
Biochemical Outcomes
The mean pretreatment PSA was 6.2 ng/ml (range 4 to 11.5). After SBRT, the mean PSA at 3- or 6-month follow-up was 1.5 ng/ml (range 0.7 to 2.4).
Discussion
The motivation for a boost to the DIL has been established, as several studies have shown the DIL to be the main site of tumor recurrence in 90% to 100% of cases.16,17 Our study investigates both the feasibility and toxicity profile of a moderate boost to the DIL in the context of SBRT for intermediate-risk prostate cancer. The feasibility and accuracy of the treatment planning technique in this work has been investigated using a patient specific 3D-printed prostate phantom and published by Lee et al.19 The results of our study demonstrate the feasibility of this planning technique in clinical practice, as the DIL received significantly higher doses than the PTV while respecting standard OAR constraints.
While our study is geared toward treatment planning technique and toxicity outcomes, it is important to consider the potential impact on clinical endpoints such as survival and biochemical control. Recently published data from the Focal Lesion Ablative Microboost in Prostate Cancer (FLAME) phase III trial show improved biochemical disease-free survival in the focal boost (up to 95 Gy to the DIL) compared with the standard arm (77 Gy in 35 fractions), with a hazard ratio of 0.45, P < 0.001.20 Although no comparable studies are published for a DIL boost in prostate SBRT, the results from the FLAME trial suggest that delivering a simultaneous integrated boost to the DIL could potentially benefit tumor control in patients with localized prostate cancer.
The efficacy and toxicity profiles associated with a boost to the DIL have also been studied for other modalities such as intensity-modulated radiation therapy (IMRT) and BT.21-29 For example, Sundahl et al found no significant difference in GU or GI toxicity with median follow-up of 72 months among patients treated with IMRT and 82-Gy simultaneous integrated boost to the DIL.21 In a prospective phase II trial, Gomez-Iturriaga et al found no grade 3 or greater toxicity at a median follow-up of 18 months among intermediate- or high-risk prostate cancer patients treated with dose escalation to the DIL via combined MRI-transrectal ultrasound fusion high-dose-rate BT.22
These results stand in contrast to studies that have investigated toxicity profiles associated with homogeneous dose escalation to the entire prostate. A phase I/II trial by Hannan et al included patients who received homogeneous SBRT of 45, 47.5 and 50 Gy in 5 fractions, and 9.8% of patients who received the 50-Gy dose had late grade 3 or greater GI toxicity.30 Within this cohort of patients, Kim et al further demonstrated that grade 3 or greater rectal toxicity was associated with > 3 cm3 volume of rectal wall receiving at least 50 Gy and > 35% rectal wall circumference receiving at least 39 Gy.31 This highlights the importance of limiting dose escalation only to the DIL and considering methods for reducing radiation dose to the rectal wall such as the use of a rectal spacer. A phase III trial conducted by Hamstra et al showed statistically significant reductions in rectal toxicity among patients undergoing IMRT with SpaceOAR, a hydrogel spacer.32 Another trial conducted by Hwang et al found that SBRT with periprostatic hydrogel placement was associated with an acute grade 1 or 2 GI toxicity rate of 16% and no recorded grade 3 or greater GI toxicity.33
The rationale for administering a moderate boost to the DIL while respecting current SBRT guidelines is to improve efficacy while minimizing toxicity by targeting the area of highest tumor activity, thus improving the therapeutic ratio. In our study, we observed a 20% incidence of acute grade 1 or 2 GI toxicity, of which 10% persisted past 90 days. We also found an 80% incidence of acute grade 1 or 2 GU toxicity, of which 50% persisted past 90 days. None of the patients developed grade 3 or greater GI or GU toxicity. However, an important consideration is the potential under-Our results can be compared with existing data on toxicity profiles for SBRT delivered at doses of 35 to 36.25 Gy without DIL dose escalation. Reported rates of acute grade 1 or 2 toxicity associated with SBRT typically fall within about 40% to 80% for GI toxicity and about 60% to 80% for GU toxicity.34-37 This is in line with the toxicity profiles from our cohort, although our acute GI toxicity rates are relatively favorable at 20%, which is comparable to the acute toxicity rate of 16% from the rectal spacer SBRT trial conducted by Hwang et al.33 Furthermore, none of the studies report grade 3 or greater GI or GU toxicities associated with SBRT given at standard doses.34-37 Based on the results of our study and comparison to treatment without dose escalation, providing a moderate boost to the DIL while following established dosimetric constraints does not appear to be associated with increased toxicity to OARs.
A recent phase I trial conducted by Herrera et al studied the toxicity associated with SBRT and simultaneous dose escalation to the DIL up to 50 Gy.18 In their trial, they found an acute grade 1 or 2 GI toxicity rate of 25%, of which 5% persisted past 90 days, and an acute grade 1 or 2 GU toxicity rate of 70%, of which 40% persisted past 90 days. None of the patients in the trial developed grade 3 or greater GI or GU toxicities. Overall, the toxicity profiles closely mirror our results. While both studies utilized dose escalation to the DIL, only our study incorporated the NRG-GU005 protocol, which establishes dosimetric constraints for SBRT treatment planning.18 Another difference between the two studies is that Herrera et al used biodegradable rectal spacers, which may have contributed to the favorable toxicity profile that they observed. However, when considered together, the two studies provide evidence that simultaneous dose escalation to the DIL, with or without the use of rectal spacers, is both safe and feasible.
An additional consideration is the impact of accurate contouring and the delivery of optimal radiation therapy that avoids underdosing the prostate while minimizing toxicity to OARs.38 This is best achieved with an interdisciplinary team and the contouring input from a diagnostic radiologist. For our study, we recruited a radiologist who assisted with contouring of the DIL for all patients. A study conducted by Dimigen et al found that advice from a consulting radiologist resulted in a change of practice in 45% of cases, ranging from changing target volumes to carrying out further imaging.39 They argue that radiologists are trained to recognize specific discrepancies from normal anatomy that a radiation oncologist, who is more concerned with encompassing CTVs, may overlook. As such, the assistance of a radiologist with formal training in image interpretation can serve as a beneficial and arguably underutilized resource in radiation therapy planning and contouring.
Limitations to our study include the relatively small sample size of 10 patients, the retrospective nature of toxicity grading, and the short follow-up period, as no patients had follow-up past 12 months. Therefore, our study does not capture radiation-induced toxicities that could potentially arise years after treatment. Future clinical trials, incorporation of larger sample sizes and longer follow-up periods could be performed to not only assess the safety and feasibility of dose escalation to the DIL, but also to examine whether dose escalation is justified by improved clinical outcomes.
Conclusion
Prostate SBRT with simultaneous moderate dose escalation to the DIL is feasible and can be accomplished while still respecting established OAR constraints. The approach to SBRT described in this study results in a favorable toxicity profile comparable to that of standard SBRT regimens without dose escalation. However, such escalation requires more specific MRI-based target delineation and likely would benefit from contouring with a radiologist.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
- Howlader N, Noone A, Krapcho M, et al. NIH National Cancer Institute. SEER Cancer Statistics Review, 1975-2016; 2019. Accessed March 1, 2020. https://seer.cancer.gov/csr/1975_2016/
- National Comprehensive Cancer Network; 2019. Prostate Cancer (Version 4.2019). Accessed March 1, 2020. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15(4):464-473. doi:10.1016/S1470-2045(14)70040-3
- Pasalic D, Kuban DA, Allen PK, et al. Dose escalation for prostate adenocarcinoma: a long-term update on the outcomes of a phase 3, single institution randomized clinical trial. Int J Radiat Oncol Biol Phys. 2019;104(4):790-797. doi:10.1016/j.ijrobp.2019.02.045
- Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol. 2018;4(6):e180039. doi:10.1001/jamaoncol.2018.0039
- Zelefsky MJ, Yamada Y, Fuks Z, et al. Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys. 2008;71(4):1028-1033. doi:10.1016/j.ijrobp.2007.11.066
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261. doi:10.1056/NEJMoa074311
- Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: / = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phy 2012;82(1):e17-24. doi:10.1016/j.ijrobp.2010.10.075
- Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52(1):6-13. doi:10.1016/s0360-3016(01)02664-5
- Dasu A, Toma-Dasu I. Prostate alpha/beta revisited — an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635
- Lukka HR, Pugh SL, Bruner DW, et al. Patient reported outcomes in NRG Oncology RTOG 0938, evaluating two ultrahypofractionated regimens for prostate cancer. Int J Radiat Oncol Biol Phys. 2018;102(2):287-295. doi:10.1016/j.ijrobp.2018.06.008
- King CR, Brooks JD, Gill H, Pawlicki T, Cotrutz C, Presti JC. Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys. 2009;73(4):1043-1048. doi:10.1016/j.ijrobp.2008.05.059
- King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109(2):217-221. doi:10.1016/j.radonc.2013.08.030
- Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: five-year outcomes. Radiat Oncol. 2011;6:3. doi:10.1186/1748-717X-6-3
- Arrayeh E, Westphalen AC, Kurhanewicz J, et al. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. Int J Radiat Oncol Biol Phys. 2012;82(5):e787-793. doi:10.1016/j.ijrobp.2011.11.030
- Cellini N, Morganti AG, Mattiucci GC, et al. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: implications for conformal therapy planning. Int J Radiat Oncol Biol Phys. 2002;53(3):595-599. doi:10.1016/s0360-3016(02)02795-5
- Herrera FG, Valerio M, Berthold D, et al. 50-Gy stereotactic body radiation therapy to the dominant intraprostatic nodule: results from a phase 1a/b trial. Int J Radiat Oncol Biol Phys. 2019;103(2):320-334. doi:10.1016/j.ijrobp.2018.09.023
- Lee CL, Dietrich MC, Desai UG, et al. A 3D-printed patient-specific phantom for external beam radiation therapy of prostate cancer. J Eng Sci Med Diagn Ther. 2018;1(4). doi:10.1115/1.4040817
- Kerkmeijer LGW, Groen VH, Pos FJ, et al. Focal boost to the intraprostatic tumor in external beam radiotherapy for patients with localized prostate cancer: results from the FLAME randomized phase iii trial. JCO. Published online January 20, 2021: JCO.20.02873. doi:10.1200/JCO.20.02873
- Sundahl N, De Meerleer G, Villeirs G, et al. Combining high dose external beam radiotherapy with a simultaneous integrated boost to the dominant intraprostatic lesion: analysis of genito-urinary and rectal toxicity. Radiother Oncol. 2016;119(3):398-404. doi:10.1016/j.radonc.2016.04.031
- Gomez-Iturriaga A, Casquero F, Urresola A, et al. Dose escalation to dominant intraprostatic lesions with MRI-transrectal ultrasound fusion high-dose-rate prostate brachytherapy. Prospective phase II trial. Radiother Oncol. 2016;119(1):91-96. doi:10.1016/j.radonc.2016.02.004
- Fonteyne V, Villeirs G, Speleers B, et al. Intensity-modulated radiotherapy as primary therapy for prostate cancer: report on acute toxicity after dose escalation with simultaneous integrated boost to intraprostatic lesion. Int J Radiat Oncol Biol Phys. 2008;72(3):799-807. doi:10.1016/j.ijrobp.2008.01.040
- De Meerleer G, Villeirs G, Bral S, et al. The magnetic resonance detected intraprostatic lesion in prostate cancer: planning and delivery of intensity-modulated radiotherapy. Radiother Oncol. 2005;75(3):325-333. doi:10.1016/j.radonc.2005.04.014
- Singh AK, Guion P, Sears-Crouse N, et al. Simultaneous integrated boost of biopsy proven, MRI defined dominant intra-prostatic lesions to 95 Gray with IMRT: early results of a phase I NCI study. Radiat Oncol. 2007;2:36. doi:10.1186/1748-717X-2-36
- Miralbell R, Mollà M, Rouzaud M, et al. Hypofractionated boost to the dominant tumor region with intensity modulated stereotactic radiotherapy for prostate cancer: a sequential dose escalation pilot study. Int J Radiat Oncol Biol Phys. 2010;78(1):50-57. doi:10.1016/j.ijrobp.2009.07.1689
- Pinkawa M, Piroth MD, Holy R, et al. Dose-escalation using intensity-modulated radiotherapy for prostate cancer - evaluation of quality of life with and without (18)F-choline PET-CT detected simultaneous integrated boost. Radiat Oncol. 2012;7:14. doi:10.1186/1748-717X-7-14
- Ares C, Popowski Y, Pampallona S, et al. Hypofractionated boost with high-dose-rate brachytherapy and open magnetic resonance imaging-guided implants for locally aggressive prostate cancer: a sequential dose-escalation pilot study. Int J Radiat Oncol Biol Phys. 2009;75(3):656-663. doi:10.1016/j.ijrobp.2008.11.023
- Uzan J, Nahum AE, Syndikus I. Prostate dose-painting radiotherapy and radiobiological guided optimisation enhances the therapeutic ratio. Clin Oncol (R Coll Radiol). 2016;28(3):165-170. doi:10.1016/j.clon.2015.09.006
- Hannan R, Tumati V, Xie X-J, et al. Stereotactic body radiation therapy for low and intermediate risk prostate cancer – results from a multi-institutional clinical trial. Eur J Cancer. 2016;59:142-151. doi:10.1016/j.ejca.2016.02.014
- Kim DWN, Cho LC, Straka C, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1-2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2014;89(3):509-517. doi:10.1016/j.ijrobp.2014.03.012
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97(5):976-985. doi:10.1016/j.ijrobp.2016.12.024
- Hwang ME, Mayeda M, Liz M, et al. Stereotactic body radiotherapy with periprostatic hydrogel spacer for localized prostate cancer: toxicity profile and early oncologic outcomes. Radiat Oncol. 2019;14(1):136. doi:10.1186/s13014-019-1346-5
- Katz AJ, Santoro M, Ashley R, Diblasio F, Witten M. Stereotactic body radiotherapy for organ-confined prostate cancer. BMC Urol. 2010;10:1. doi:10.1186/1471-2490-10-1
- Chen LN, Suy S, Uhm S, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol. 2013;8:58. doi:10.1186/1748-717X-8-58
- Park Y, Park HJ, Jang WI, Jeong BK, Kim H-J, Chang AR. Long-term results and PSA kinetics after robotic SBRT for prostate cancer: multicenter retrospective study in Korea (Korean radiation oncology group study 15-01). Radiat Oncol. 2018;13(1):230. doi:10.1186/s13014-018-1182-z
- McBride SM, Wong DS, Dombrowski JJ, et al. Hypofractionated stereotactic body radiotherapy in low-risk prostate adenocarcinoma: preliminary results of a multi-institutional phase 1 feasibility trial. Cancer. 2012;118(15):3681-3690. doi:10.1002/cncr.26699
- Salembier C, Villeirs G, De Bari B, et al. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother Oncol. 2018;127(1):49-61. doi:10.1016/j.radonc.2018.01.014
- Dimigen M, Vinod SK, Lim K. Incorporating a radiologist in a radiation oncology department: a new model of care? Clin Oncol (R Coll Radiol). 2014;26(10):630-635. doi:10.1016/j.clon.2014.04.030
Citation
Yu S, Huang D, Mathew JS, Dyer MA, Bloch BN, Keohan S, Hirsch AE. SBRT With Integrated Boost to the Dominant Intraprostatic Nodule: Initial Dosimetric and Clinical Outcomes. Appl Rad Oncol. 2021;(1):36-42.
March 30, 2021
