Stereotactic body radiotherapy for the treatment of spine metastasis
Images
CASE SUMMARY
A 56-year-old female with renal cell carcinoma (RCC) diagnosed 7 months prior to presentation was evaluated for severe left-sided back pain originating in the left anterior hip with radiation down the left leg. She reported parasthesias along with the pain, which was present at rest and worsened with movement. She denied any associated bowel or bladder dysfunction, saddle anesthesia, or gait difficulties. At presentation, she was on maintenance Sunitinib and had an increasing narcotic requirement.
IMAGING FINDINGS
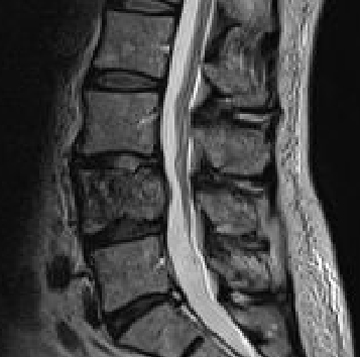
Magnetic resonance imaging (MRI) with and without intravenous contrast demonstrated loss of L4 vertebral height and replacement of the L4 vertebral body with a contrast-enhancing heterogenous expansile mass, which extended into the left pedicle. Mild extraosseous epidural soft tissue as well as moderate narrowing of the L4-5 neural foramen was also noted (Figure 1).
DIAGNOSIS
Spine metastasis from renal cell carcinoma
DISCUSSION
Spine is the third most frequent site of metastasis, and approximately 10% of all cancer patients develop symptomatic spinal metastasis. About 10% to 20% of patients with spine metastasis will develop spinal cord compression.1 The majority (70%) of spinal metastases are located in the thoracic spine and approximately half of all patients have multiple spinal metastases. Patients with spine metastasis can present clinically with pain and/or neurologic dysfunction. In patients with neurologic dysfunction, it is imperative to rule out spinal cord compression. The preferred imaging modality for spine imaging is MRI, however, in patients with contraindications to MRI, a computed tomography (CT) scan or a myelogram would be acceptable. Prompt spine imaging also helps in establishing the stability of the spine.
Initial management of spinal metastasis involves the use of narcotic and non-narcotic pain medications. However, if spine instability or spinal cord compressions are present, surgical decompression and spinal stabilization are mainstays of therapy. Radiotherapy, either conventionally fractionationated external beam radiotherapy (CRT) or spine stereotactic body radiotherapy (sSBRT) can also be utilized for the treatment of spinal metastasis.
One of the main goals of palliative radiotherapy to the spine is pain control.2 For many decades, CRT has been utilized. Because the treatment field involves the spinal cord, CRT can be delivered safely up to 2 times before the risk of myelopathy outweighs the potential benefits.3 With the advancement of chemotherapy regimens and the development of targeted chemotherapeutics, patients with metastatic RCC have longer survival, which necessitates more durable pain control.4 Furthermore, patients who have undergone prior CRT without significant pain relief or patients who have received prior incidental spinal radiation secondary to treatment for their primary cancer (such as lung cancer or anal cancer) are poor candidates for CRT for spinal metastasis.
Over the past 2 decades, sSBRT has been developed and refined, allowing for the delivery of high dose conformal radiotherapy to the spine, while adequately sparing the spinal cord. Importantly, sSBRT enables the delivery of a higher biologically effective dose (BED) than CRT does. Although differences in technique exist between institutions, here we will discuss the approach utilized at the Cleveland Clinic, which is congruous with the approach of the ongoing RTOG 0631 trial.5 We refer the interested reader to a recent review for a detailed discussion of institutional differences.6
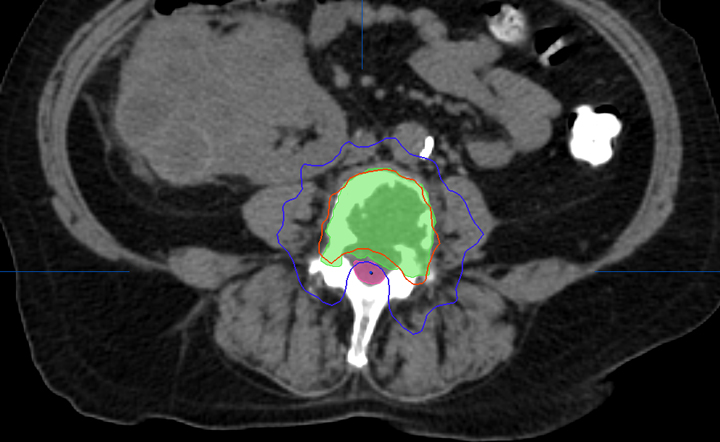
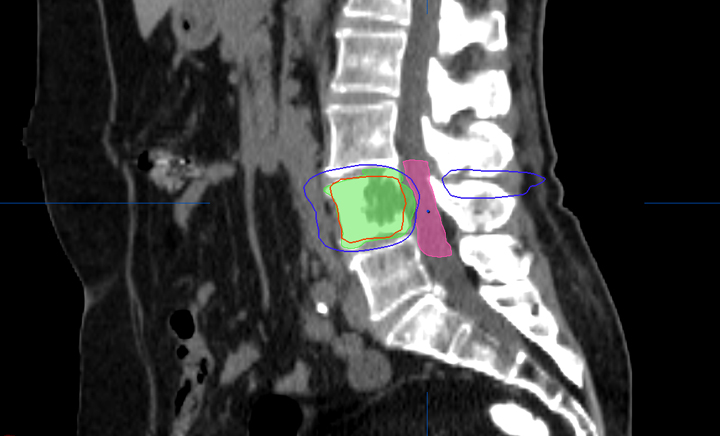
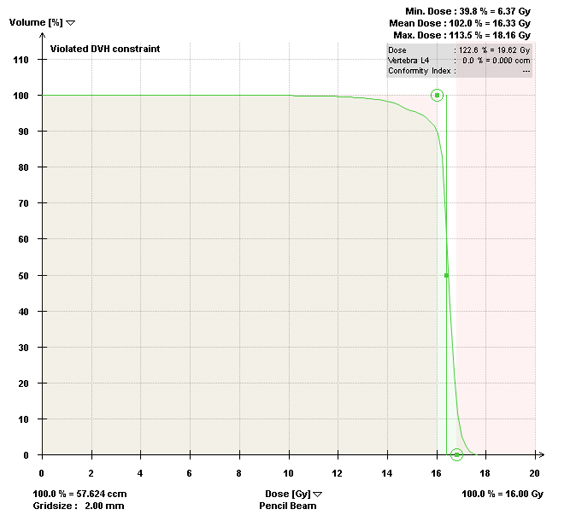
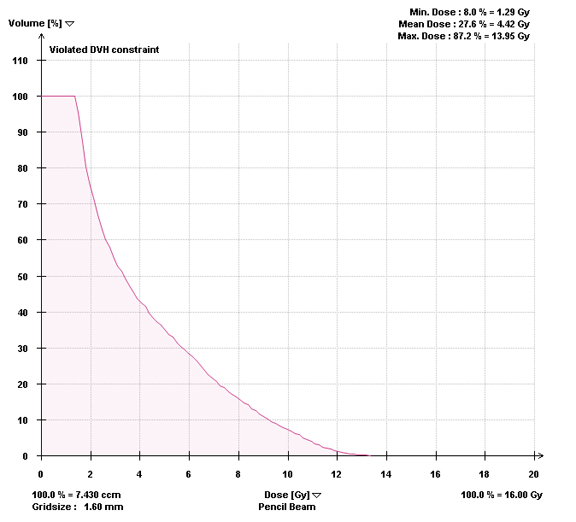
Most centers utilize either a single-fraction or a hypofractionated regimen. At Cleveland Clinic, most patients are treated with either 16Gy or 18Gy in 1 fraction with the higher dose reserved for radioresistant histologies. For treatment planning, we limit the spinal cord to a maximum dose < 14Gy and 10% of spinal cord receiving ≤ 10Gy and limit the cauda equina to a maximum dose < 16Gy and 10% of cauda equina receiving ≤ 12Gy (Figures 2 and 3). With either the single fraction or hypofractionated regimens, most centers have reported local control rates ≥ 80%.6
Nguyen et al studied sSBRT in 48 patients with 55 RCC spinal metastases treated with either 24Gy in 1 fraction, 27Gy in 3 fractions, or 30Gy in 5 fractions and demonstrated a local control rate of 82.1% and a complete pain response of 44% and 52% at 1 month and 12 months post-sSBRT, respectively.7 We recently reported our results for 57 patients with 88 spinal metastases from RCC and demonstrated a complete pain response of 27.1% and 63.6% at 1 month and 12 months post-sSBRT, respectively. Multivariate analysis demonstrated that multilevel disease and neural foramen involvement were associated with radiographic failure and multilevel disease and pre-existing vertebral body fracture were associated with pain failure.8 Hunter et al recently compared the efficacy of CRT versus sSBRT for patient RCC spinal metastases. He showed that sSBRT offered more complete pain relief (33% vs 12%, p=0.01), whereas CRT offered more partial pain relief (56% vs 29%, p=0.01). A trend towards longer duration of pain relief with sSBRT was also demonstrated (4.8m vs 1.7m, p=0.05).4 These differences may be attributable to the higher BED delivered by sSBRT compared to CRT.
The most common acute toxicities from sSBRT include fatigue (up to 40%) and gastrointestinal effects (10-20%).9 Major long-term toxicities include the risk of developing vertebral body fractures as well as the risk of spinal cord myelopathy.
Rose et al evaluated 62 consecutive patients with 71 vertebral bodies treated with 18Gy to 24Gy in a single fraction and reported a 39% incidence of new or progressive vertebral fractures. Risk factors for fractures were location (T10 to sacrum), lytic metastasis and > 40% vertebral body involvement.10 A multi-institutional study by Sahgal et al of 254 patients with 410 vertebral levels found a fracture incidence of 14% and identified dose per fraction (> 20Gy), preexisting vertebral fracture, lytic metastasis presence of paraspinal, and/or epidural disease and spinal deformity as risk factors for developing a vertebral fracture post-sSBRT (Sahgal et al, provisionally accepted to Journal of Clinical Oncology).
Risk of spinal cord myelopathy from sSBRT is estimated to be < 1%.3 Sahgal et al performed a multi-institutional study of 5 myelopathy cases comparing it to 19 unaffected patients post-sSBRT. His analysis demonstrated that thecal-sac maximum doses of up to 10Gy in a single fraction were safe. Furthermore, using BED calculations, he demonstrated that 30 to 35 2-Gy equivalent for up to 5 fractions was also safe.11 Aggregating all the available clinical reports of spinal-cord tolerance in the setting of sSBRT, Kirkpatrick et al concluded that 13Gy in 1 fraction or 20Gy in 3 fractions confers a spinal-cord myelopathy risk of < 1%.3
CONCLUSION
Spine SBRT is now a well-established technique for the treating spinal metastasis that has enhanced the treatment of patients with spine metastasis. Many studies have shown that sSBRT provides excellent local control and is safe and effective in the primary as well as salvage settings. sSBRT provides rapid and durable pain relief and can also be utilized in patients that have significant epidural disease. Currently, sSBRT remains the only effective nonsurgical option in patients previously irradiated for spinal metastases. sSBRT is not only a convenient option for patients as it can be delivered in one setting but it also has a low risk of acute and late toxicities.
REFERENCES
- Eleraky M, Papanastassiou I, Vrionis FD. Management of metastatic spine disease. Curr. Opin Support Palliat Care. 2010;4:182-188.
- Hartsell WF, Scott CB, Bruner DW, et al. Randomized Trial of Short- Versus Long-Course Radiotherapy for Palliation of Painful Bone Metastases. Jnci J Natl Cancer Inst. 2005;97:798-804.
- Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76: S42-49.
- Hunter GK, Balagamwala EH, Koyfman SA, et al. The efficacy of external beam radiotherapy and stereotactic body radiotherapy for painful spinal metastases from renal cell carcinoma. Pr Radiat Oncol. 2012;2:e95-e100.
- Anon. RTOG 0631 Phase II/III Spine Mets.
- Balagamwala EH, Cherian S, Angelov L, et al. Stereotactic body radiotherapy for the treatment of spinal metastases. J Radiat Oncol. 2012;1: 255-265.
- Nguyen Q-N, Shiu AS, Rhines LD, et al. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1185-1192.
- Balagamwala EH, Angelov L, Koyfman SA, et al. Single-fraction stereotactic body radiotherapy for spinal metastases from renal cell carcinoma. J Neurosurg. Spine. 2012;17:556-564.
- Masucci GL, Yu E, Ma L, et al. Stereotactic body radiotherapy is an effective treatment in reirradiating spinal metastases: current status and practical considerations for safe practice. Expert Rev Anticancer Ther. 2011;11:1923-1933.
- Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:5075-5079.
- Sahgal A, Ma L, Gibbs I, et al. Spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:548-553.