Stereotactic radiosurgery for cerebellar metastases and the risk of obstructive hydrocephalus
Images
Abstract
Purpose: Cerebellar location has been reported as a poor prognostic factor among patients with brain metastases. Stereotactic radiosur gery (SRS) is commonly utilized in patients with brain metastases, but the role of SRS in cerebellar metastases is less clear. It is believed that SRS could result in obstructive hydrocephalus with tumor edema or progression, and resection could minimize that risk. Our purpose was to report our institution’s clinical experience treating such patients to investigate this concern.
Methods: Patients with brain metastases treated with SRS to cerebellar disease at their first SRS session at our institution from 1997 to 2014 were included in the analysis. Patient and tumor characteristics, dosimetry, toxicity, and survival following SRS were collected and analyzed for factors associated with obstructive hydrocephalus and SRS toxicity.
Results: One hundred patients with 155 cerebellar metastases met inclusion criteria. The median Karnofsky performance score (KPS) was 90 and median number of cerebellar metastases was 1 (range: 1 to 7). Prior cerebellar tumor resection was performed in 9.7% (n = 15) of tumors, and whole-brain irradiation in 30% (n = 30) of patients. Patients received a median SRS prescription of 20 Gy to the 50% isodose line. Median overall survival was 15.9 months, and 4 patients (4%) developed obstructive hydrocephalus and/or received a shunt following their first SRS. On multivariate analysis, after controlling for tumor volume and proximity to the 4th ventricle, the only factor associated with probability of developing hydrocephalus and/or shunt placement after SRS was previous resection (p = 0.02).
Conclusion: Our series demonstrates that SRS to cerebellar metastasis is generally safe and effective. Resection prior to SRS may increase the long-term risk for subsequent obstruction. While cerebellar tumor location may be associated with poor prognosis, SRS-related toxicity is uncommon.
Intracranial metastases are a sign of systemic progression in patients with a malignancy and have a dismal prognosis if left untreated.1 While limited brain metastases have been traditionally managed with open neurosurgical resection,2 in recent years stereotactic radiosurgery (SRS) has emerged as a widely accepted therapeutic option for patients with limited brain metastases.3-7 Unless extirpation is indicated for immediate relief from mass effect symptoms or for histopathologic diagnosis, SRS is less invasive and preferred when treating multiple, small metastases, especially when eloquent or deep-seated areas are involved.
While most metastases occur in the supratentorium, the posterior fossa accounts for about 15% to 20% of tumors.8,9 Compared to supratentorial location, metastasis to the cerebellum has been reported as a negative prognostic factor for survival.10,11 One potential explanation is that, by virtue of its location, patients with cerebellar metastases are at risk for obstructive hydrocephalus and brainstem compression.8,12-14 However, only a few series in the literature evaluate the outcomes of SRS specific to cerebellar metastases.14,15 Although survival outcomes have been reported, there were limited data to determine rates of toxicity following treatment with SRS.
The purpose of this investigation was to evaluate our institution’s experience in treating patients with cerebellar metastases with SRS, including the efficacy and toxicity outcomes of cerebellar SRS, with the goal of addressing the theoretical concern that SRS to the cerebellum can result in post-SRS obstructive hydrocephalus.
Methods
Patient Population
Data were obtained through a prospectively collected, IRB-approved database of patients treated with SRS at our institution. All patients received SRS for cerebellar metastases between 1997 and 2014 on a Leksell Gamma Knife radiosurgery platform (Elekta, Stockholm, Sweden). Patients receiving cerebellar surgical resection, whole-body radiation therapy (WBRT), and/or chemotherapy prior to receiving SRS were included, although patients who underwent SRS to other lesions and later received cerebellar SRS were excluded. Clinical characteristics for each patient were collected including gender, age, Karnofsky performance score (KPS), tumor histology, maximal cerebellar tumor diameter, cerebellar tumor volume, intracranial and extracranial disease burden, prior therapies, and overall survival. Distance to the 4th ventricle was calculated by measuring the shortest linear distance (mm) from the tumor to the 4th ventricle on an axial-oriented MRI (T1 gadolinium-enhanced sequence). SRS dosimetric parameters including margin dose, isodose, and maximum dose were additionally collected through review of the treatment planning software.
Stereotactic Radiosurgery
Our institution’s SRS technique has been described previously.16,17 In brief, patients are treated with a Leksell Gamma Knife radiosurgery unit in a single fraction. Three Gamma Knife models were used during this period: the model U (1992-2001), the model C (2001-2007), and the Perfexion (2007-present). After stereotactic head frame placement and neuroimaging (MRI and/or CT if MRI is medically contraindicated), the images were loaded to the radiosurgical planning software and coregistered in 3-dimensional space to the SRS treatment platform. Prior to treatment initiation, all radiosurgical plans were reviewed and approved by a neurosurgeon, radiation oncologist, and medical physicist. Utilizing 60 Co sources, the prescribed dose was delivered via one or more isocenters to the isodose line encompassing the periphery of the tumor defined by neuroimaging (without margin). In the postoperative setting, our general practice is to target the postoperative cavity as defined by MRI and prescribe coverage that extends 1mm into the adjacent tissue. We also cover any enhancing tumor at the operative bed. Of note, we typically wait 1 to 2 weeks between resection and SRS for dynamic changes at the tumor resection cavity to be largely stabilized.18
Follow-up
Patients typically underwent imaging follow-up at approximately 3-month intervals after SRS. All images were reviewed by treating clinicians and a neuroradiologist. These images were reviewed and compared to images obtained at the time of SRS for changes in tumor volume (increase, decrease, or stable) and brain edema (increase, decrease, or stable). Tumor volume growth > 10% during follow-up were considered evidence of local failure.19 Patients had regular clinical follow-up after SRS to monitor for clinical toxicity and/or progressive disease. These medical records were reviewed for evidence of new or worsening cerebellar mass effects (imbalance, ataxia, nausea, and vomiting), hydrocephalus, shunt insertion after SRS, new cranial nerve deficits, and adverse effects from treatment (ie, SRS-induced edema requiring steroids) graded by the Common Terminology Criteria for Adverse Events version 4.20 Patients who underwent ultimate resection of the cerebellar metastasis after SRS were also recorded. Overall survival (OS) was measured from the data of SRS to the date of death or last follow-up.
Statistical Analyses
Potential prognostic variables (eg, age, gender, laterality, histology, KPS, total number of brain and cerebellar metastases, tumor volume, distance to the 4th ventricle, margin dose, isodose, maximum dose, and pre-SRS interventions) were evaluated using logistic regression models for an association with hydrocephalus and/or shunt placement following cerebellar SRS. Overall survival (OS) and local control (LC) were analyzed using the Kaplan-Meier method. All statistical analyses in this study were performed using statistical software (SPSS, version 20.0; SPSS Inc., Chicago, Illinois).
Results
Clinical Outcomes
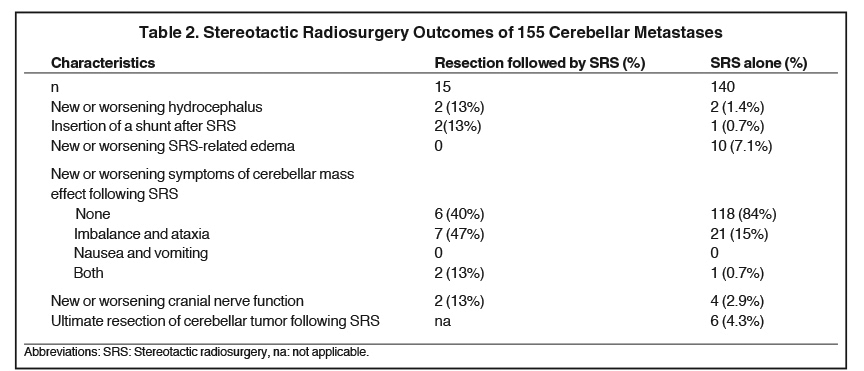
We identified 100 patients with 155 cerebellar metastases treated with SRS at our institution. The clinical characteristics of the cohort are summarized in Table 1. The median KPS at the time of SRS was 90 (range: 60 to 100). Cerebellar tumor resection was performed prior to SRS in 15 patients (9.7%). The number of cerebellar metastases ranged from 1 to 7 (median: 1 cerebellar tumor). The cerebellar metastases had a median diameter of 8 mm (range: 0.1 - 50.0 mm) and the median distance to the 4th ventricle was 19 mm (range: 0 - 46 mm). The typical SRS prescription delivered 20 Gy into the 50% isodose line. Most “other” tumor histologies were of gastrointestinal origin (38 tumors, 24.5%).
Overall Survival and Local Control
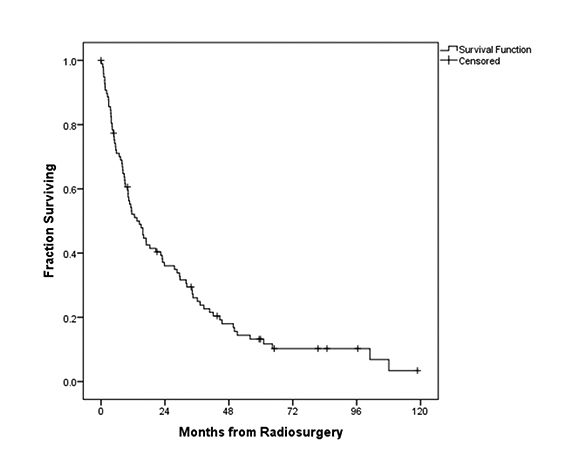
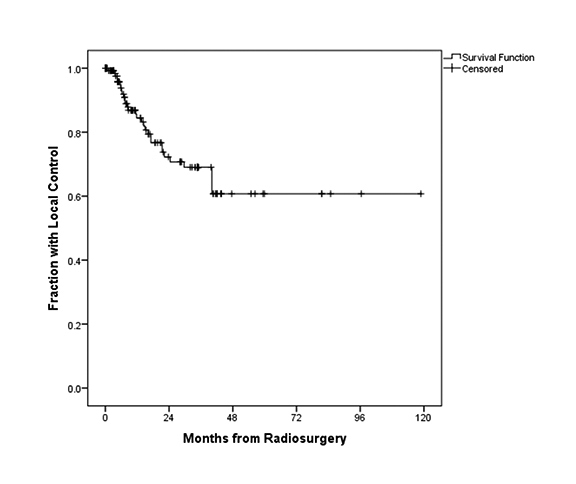
In this series, median imaging follow-up and OS were 14.8 months and 15.9 months, respectively. Figure 1 illustrates the OS for all patients, with a median OS of 15.9 months. In terms of local control, Figure 2 demonstrates the fraction of patients with local control during follow-up. Local control following SRS was 99%, 93%, and 84% at 3, 6, and 12 months, respectively.
Clinical Outcomes
Table 2 summarizes the clinical outcomes of patients in this series, stratified by whether they underwent pre-SRS resection or SRS alone. As demonstrated in Table 2, new or worsening hydrocephalus occurred more frequently in the pre-SRS resection cohort (13% vs. 1.4%). Onset of new/worsening cerebellar mass effect symptoms was also more frequent in the pre-SRS resection cohort, as was the relative rate of cranial nerve deficits (Table 2). Interestingly, SRS-related edema was not observed with the pre-SRS resection cohort (vs. 10 % in the SRS alone cohort).
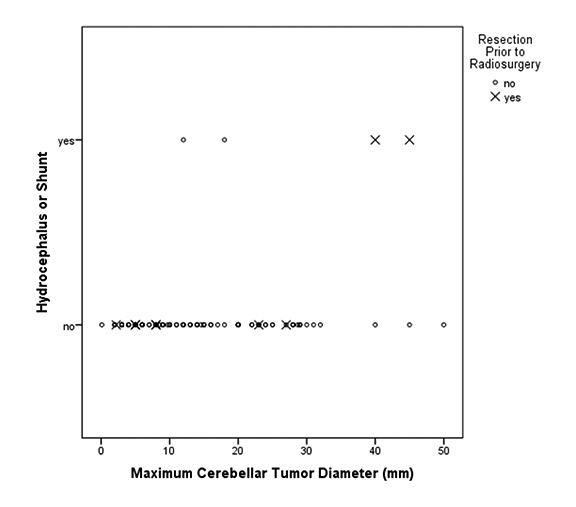
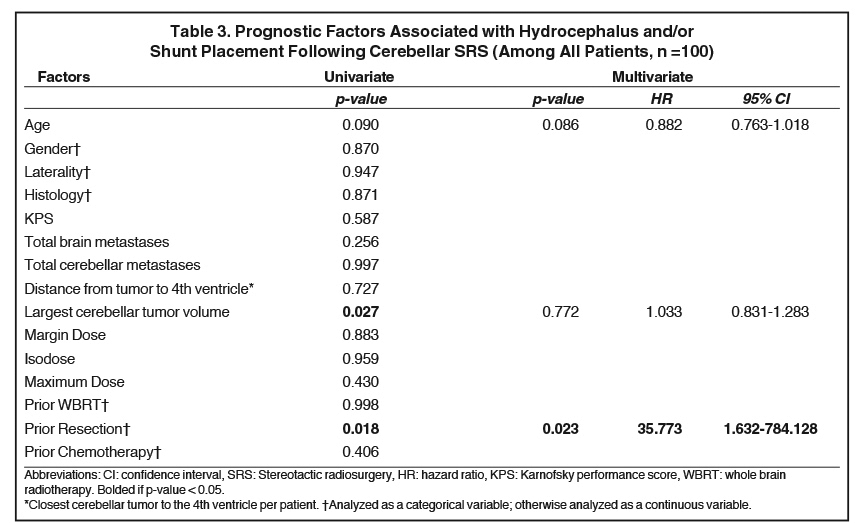
Figure 3 depicts the relationship between cerebellar tumor size and subsequent hydrocephalus and/or shunt. Table 3 displays the prognostic factors associated with hydrocephalus and/or shunt placement following cerebellar SRS by both univariate and multivariate analyses. Of the investigated variables, only resection prior to SRS was associated with a significantly increased risk of hydrocephalus and/or shunt placement status after SRS (HR 35.8, 95%CI 1.6 - 784.13, p = 0.023). Cerebellar tumor volume, cerebellar tumor number, distance to the 4th ventricle, margin dose, isodose, and prior WBRT failed to demonstrate an association with the ultimate development of obstructive hydrocephalus.
Discussion
Cerebellar metastases are unique due to the reported risk of obstructive hydrocephalus and intracranial hypertension,8,12-14 and their reported association with inferior survival outcomes.10,11 However, limited data exist regarding treatment outcomes after radiation therapy for cerebellar metastases, with only a few published series to date.14,15,21 Our study demonstrates that SRS can be safely used for cerebellar metastases and, interestingly, that resection prior to SRS could be associated with a higher risk of toxicity following SRS. As the paradigm has shifted from WBRT to SRS for limited intracranial disease, further understanding of the contribution of less-studied clinical factors for SRS such as tumor location is critical for therapeutic decision-making.
This analysis has a unique position within the context of the existing literature. In an analysis by Javalkar et al of 35 patients with solitary, small cerebellar metastases, 24 were treated with resection and adjuvant WBRT and 11 had SRS alone.14 Local failure, distant failure, and overall survival were not statistically significantly different between patients treated with resection and those not.14 In contrast, Ampil et al found that median OS was higher in patients receiving surgery and adjuvant WBRT (15 months) compared to WBRT alone (3 months).21 However, this could have been confounded by the difference in the number of brain metastases between groups (15% of the WBRT alone group had solitary cerebellar metastasis compared to 73% in the group receiving resection first, p = 0.001).21 A larger series of 109 patients found that survival outcomes were best after surgery and adjuvant RT, including WBRT or SRS, (35.5 months) compared to resection alone (20.5 months), WBRT alone (6.5 months), or SRS alone (9.1 months).15 The results from this study are difficult to interpret as baseline prognostic features were reported based on the entire series not on a cohort-by-cohort basis. Fadul et al determined that WBRT and resection yielded a median OS of 6 months compared to 5.5 months for WBRT alone, although the sample size was relatively small (n = 21).13
Our series is unique in that it is the first to report on treatment toxicity rates following SRS for metastatic disease to the cerebellum and, in addition, identifies significant prognostic factors. As a historical barometer, RTOG 90-05, a phase I dose escalation trial, evaluated SRS dose tolerance limits in previously irradiated tumors and determined that SRS has an acceptable safety profile, although only among patients with gross disease.22 The results of our series support this conclusion in our patient cohort, as the overall incidence of toxicity following SRS was low. When subjected to multivariate analyses, the only factor significantly associated with post-SRS hydrocephalus and/or shunt placement was previous surgical resection. Of note, other factors such as total cerebellar metastases, largest tumor volume, and even distance to the 4th ventricle failed to demonstrate a relationship. A reasonable assumption is that patients initially treated with surgery prior to receiving SRS had larger tumors with impending ventricular obstruction, although we could not identify that association. It is also possible that previously resected tumors have more risk of debris (eg, hemorrhage or cells from piecemeal resection of the tumor), which could cause unintended consequences of cerebrospinal fluid (CSF) outflow obstruction over time.
In light of our study results, perhaps a more plausible explanation is that SRS induces inflammation and this is compounded with changes already brought about by surgery, precipitating extensive peritumoral edema that can compress the adjacent ventricles. In contrast, we failed to identify SRS-induced edema in the pre-SRS resection cohort, a finding that could be related to the difficulty interpreting postoperative and post-SRS parenchymal changes radiographically. Patel et al reviewed MRI sequences of 516 brain metastases treated with SRS and determined that 32% of lesions increased in volume following SRS with a delayed onset often emerging 6 weeks following treatment and lasting as long as 15 months post-SRS.23 They concluded that post-SRS growth was not always due to tumor recurrence but can be a sign of an inflammatory response. Hypothetically, cellular damage and the release of inflammatory toxins could lead to a pronounced inflammatory reaction, increased vasogenic edema, and a breakdown of the blood-brain barrier. Furthermore, studies on intracranial meningomas have reported on the importance of the tumor-brain contact interface area and how disruption of the interface is a prognostic factor for peritumoral edema.24 One can extrapolate this data to suggest that extensive neural damage caused by resection and radiosurgery combined can also create architectural changes that predispose a patient to similar outcomes. In addition, brain metastases are already at an increased risk of intracranial edema due to disruption of the blood-brain barrier and increased permeability of tumor vessels.25-27 Although distance to the 4th ventricle was not a significant prognostic factor, it bears considering that cerebellar metastases likely have a higher inherent risk for hydrocephalus given the proximity to the ventricular system compared to their supratentorial counterparts.8,12-14 The combination of these factors – location, inherent predisposition to inflammation, and combined modality therapy – could produce a cascade of events increasing the risk for a mass effect on the ventricular system following treatment with SRS.
The limitations of this study relate to this being a retrospective analysis of a single institution’s experience with the utilization of radiosurgery for cerebellar metastases. However, the size of the cohort makes this study a significant contribution to the literature. It is not certain that these results are generalizable to other radiation techniques, such as fractionated stereotactic radiation therapy.28 In this series, 30% of patients had prior WBRT and 9.7% of patients had prior resection, so we could not isolate complications solely due to SRS. However, the current series reflects the multimodality treatment now commonly employed to treat brain metastases. Although we attempted to control for factors associated with the risk of hydrocephalus, the factors associated with the decision for neurosurgical tumor resection are complicated and could not be completely controlled for our analysis. In the future, similar analyses incorporating conformality indices will be critical in defining the drivers of toxicity in these patients.
Conclusion
In the first study to describe treatment toxicity rates following the use of radiosurgery for metastatic disease in the cerebellum, prior intervention with resection was associated with an increased rate of toxicity following SRS. However, the overall incidence of treatment toxicity was low, demonstrating that SRS is a safe treatment option for cerebellar metastases even following surgical resection. Although further studies are needed to compare outcomes with different treatment modalities, our survival rates with SRS are encouraging. These data suggest that radiosurgery monotherapy (without resection) has the potential to result in acceptable toxicity, local control, and favorable survival rates in the cerebellum in properly selected patients. Future studies on radiosurgery for brain metastases should consider intracranial tumor location in clinical factor stratification due to the potential of location-specific mortality and morbidity effects.
References
- Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys.1999;43:795-803.
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
- Sneed PK, Suh JH, Goetsch SJ, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metasta
- ses. Int J Radiat Oncol Biol Phys. 2002;53:519-526.
- Sanghavi SN, Miranpuri SS, Chappell R, et al. Radiosurgery for patients with brain metastases: a multi-institutional analysis, stratified by the RTOG recursive partitioning analysis method. Int J Radiat Oncol Biol Phys. 2001;51:426-434.
- Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486-2493.
- Hall MD, McGee JL, McGee MC, et al. Cost-effectiveness of stereotactic radiosurgery with and without whole-brain radiotherapy for the treatment of newly diagnosed brain metastases. J Neurosurg. 2014;121(suppl):84-90.
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-2491.
- Weisberg LA. Solitary cerebellar metastases. Clinical and computed tomographic correlations. Arch Neurol. 1985;42:336-341.
- Delattre JY, Krol G, Thaler HT, et al. Distribution of brain metastases. Archives of neurology. 1988;45:741-744.
- Wronski M, Arbit E. Resection of brain metastases from colorectal carcinoma in 73 patients. Cancer. 1999;85:1677-1685.
- Chaichana KL, Rao K, Gadkaree S, et al. Factors associated with survival and recurrence for patients undergoing surgery of cerebellar metastases. Neurol Res. 2014;36:13-25.
- Ghods AJ, Munoz L, Byrne R. Surgical treatment of cerebellar metastases. Surg Neurol Int. 2011;2:159.
- Fadul C, Misulis KE, Wiley RG. Cerebellar metastases: diagnostic and management considerations. J Clin Oncol. 1987;5:1107-1115.
- Javalkar V, Cardenas R, Ampil F, et al. The Louisiana State University experience in the management of single small cerebellar metastasis. Neurosurgery. 2010;67:1515-1522.
- Yoshida S, Takahashi H. Cerebellar metastases in patients with cancer. Surg Neurol. 2009;71:184-187; discussion 187.
- Trifiletti DM, Lee CC, Schlesinger D, et al. Leukoencephalopathy after stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2015;93:870-878.
- Trifiletti DM, Lee CC, Winardi W, et al. Brainstem metastases treated with stereotactic radiosurgery: safety, efficacy, and dose response. J Neurooncol. 2015;125:385-392.
- Jarvis LA, Simmons NE, Bellerive M, et al. Tumor bed dynamics after surgical resection of brain metastases: implications for postoperative radiosurgery. Int J Radiat Oncol Biol Phys. 2012;84:943-948.
- Snell JW, Sheehan J, Stroila M, et al. Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error. Technical note. J Neurosurg. 2006;104:157-162.
- National Insitutes of Health NCI, Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed July 22, 2015.
- Ampil FL, Nanda A, Willis BK, et al. Metastatic disease in the cerebellum. The LSU experience in 1981-1993. Am J Clin Oncol. 1996;19:509-511.
- Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291-298.
- Patel TR, McHugh BJ, Bi WL, et al. A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. Am J Neuroradiol. 2011;32:1885-1892.
- Cai R, Barnett GH, Novak E, et al. Principal risk of peritumoral edema after stereotactic radiosurgery for intracranial meningioma is tumor-brain contact interface area. Neurosurgery. 2010;66:513-522.
- Wen PY, Schiff D, Kesari S, et al. Medical management of patients with brain tumors. J Neurooncol. 2006;80:313-332.
- Papadopoulos MC, Saadoun S, Binder DK, et al. Molecular mechanisms of brain tumor edema. Neuroscience. 2004;129:1011-1020.
- Strugar J, Rothbart D, Harrington W, et al. Vascular permeability factor in brain metastases: correlation with vasogenic brain edema and tumor angiogenesis. J Neurosurg. 1994;81:560-566.
- Minniti G, Scaringi C, Paolini S, et al. Single-fraction versus multifraction (3 x 9 gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016.15;95(4):1142-1148.
Citation
Hill C, Trifiletti DM, Romano KD, Showalter TN, Sheehan JP . Stereotactic radiosurgery for cerebellar metastases and the risk of obstructive hydrocephalus. Appl Rad Oncol. 2017;(1):17-23.
March 8, 2017
