Telix to Start Phase 1 Clinical Trial of Prostate Cancer Imaging Product in Japan
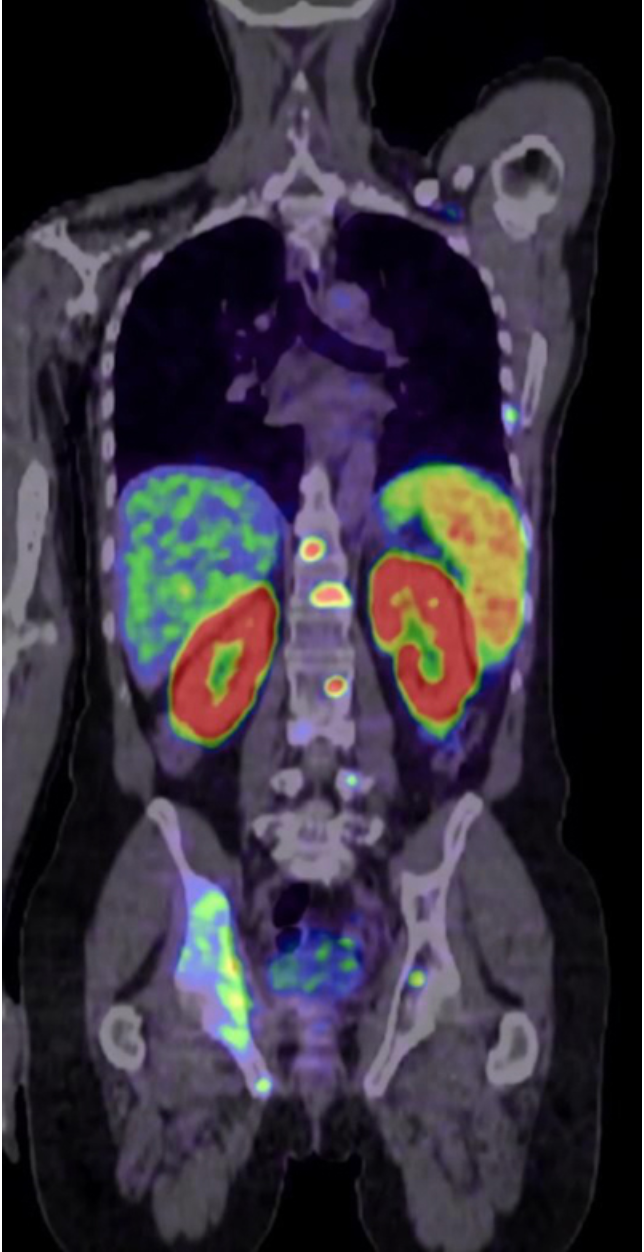 Telix Pharmaceutical Japan KK, a subsidiary of Telix Pharmaceuticals, has announced receipt of Clinical Trial Notification clearance by the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) to begin its Phase I trial of its prostate cancer imaging product TLX591-CDx (Kit for the preparation of 68Ga-PSMA-11 injection) in Japan. The company is collaborating with Kanazawa University on the clinical trial.
Telix Pharmaceutical Japan KK, a subsidiary of Telix Pharmaceuticals, has announced receipt of Clinical Trial Notification clearance by the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) to begin its Phase I trial of its prostate cancer imaging product TLX591-CDx (Kit for the preparation of 68Ga-PSMA-11 injection) in Japan. The company is collaborating with Kanazawa University on the clinical trial.
“68Ga-PSMA-11 has been studied widely and intensively across the globe outside of Japan. Clinical data have been accumulated and its diagnostic efficacy and clinical application have been well characterized in guidelines in nuclear medicine and urology, worldwide,” said Professor Seigo Kinuya, Professor of Nuclear Medicine at the Kanazawa University Hospital, Department of Nuclear Medicine, Chair of the Japanese Society of Nuclear Medicine, and Chair of the National Conference for Nuclear Medicine Theranostics. “This first clinical trial of 68Ga-PSMA-11 prostate imaging in Japan is a crucial milestone to pave the way to provide state-of-the-art diagnostics to Japanese prostate cancer patients."
Telix and Kanazawa University previously collaborated on a Phase I trial of TLX591-CDx in 10 patients with advanced prostate cancer in December 2020. The trial will obtain preliminary clinical data in a suitable patient population, confirming that the targeting and pharmacology of TLX591-CDx is equivalent to non-Japanese patients. The clinical data will support future planning discussions with the objective of PMDA product approval in Japan, aligning with the marketing authorizations that Telix has already submitted in the US, EU, Canada and Australia.
Telix Pharmaceuticals Japan K.K. President Dr. Shintaro Nishimura added, “This study is the first formal clinical trial in Japan of a gallium 68-labeled PET imaging agent, which many Japanese nuclear medicine and urology physicians have been waiting for. We would like to express our appreciation to our pioneering colleagues at Kanazawa University’s Departments of Nuclear Medicine and Urology, the Innovative Clinical Research Center of Kanazawa University Hospital, the Kanazawa Advanced Medical Center, ATOX Co. Ltd and IRE ELiT (Belgium) for their exceptional support and collaboration in this project.”