The Evidence and Rationale for a Coronary Brachytherapy Dose-Response
Images


Abstract
Background: Multiple prospective, randomized trials have confirmed that vascular brachytherapy can prevent in-stent restenosis (ISR) after percutaneous coronary intervention (PCI). Although several observational studies suggest short-term effectiveness, the rate of long-term ISR after salvage intravascular brachytherapy (IVBT) is approximately 40% at 3 years’ follow-up. While moderately effective, there is clearly room to improve IVBT.
Methods: We used the PubMed search engine with the terms coronary, intravascular brachytherapy, dose, and response.
Results: A positive dose-response relationship has been shown for IVBT, based on preclinical, retrospective and prospective randomized clinical trials. There has been remarkably little toxicity of IVBT, despite many thousands of patients being treated on and off trials. Considering the lack of reported complications despite meticulous follow-up of hundreds of patients enrolled in studies, it seems that coronary vessel tolerance to radiation may be higher than the current prescription doses.
Conclusion: Given the high rate of failure in patients with recurrent ISR, and a fairly consistent dose-response relationship in most studies, further clinical investigation of higher prescription doses seems warranted.
Keywords: radiation, oncology, coronary, brachytherapy, heart, cardiac, intravascular
Radiation is routinely used for a variety of noncancerous conditions, including keloids, heterotopic bone formation, Dupuytren’s contracture, and arteriovenous malformations.1-4 Its use against atherosclerotic vascular disease was first explored in the 1960s.5 By the 1990s, intravascular brachytherapy (IVBT) techniques were developed to prevent restenosis after coronary angioplasty or stent placement.6 At least 6 prospective, randomized trials have confirmed the clinical efficacy of IVBT, which reduces the restenosis rate from approximately 40% to 20%, depending on patient characteristics and the length of follow-up.7-12
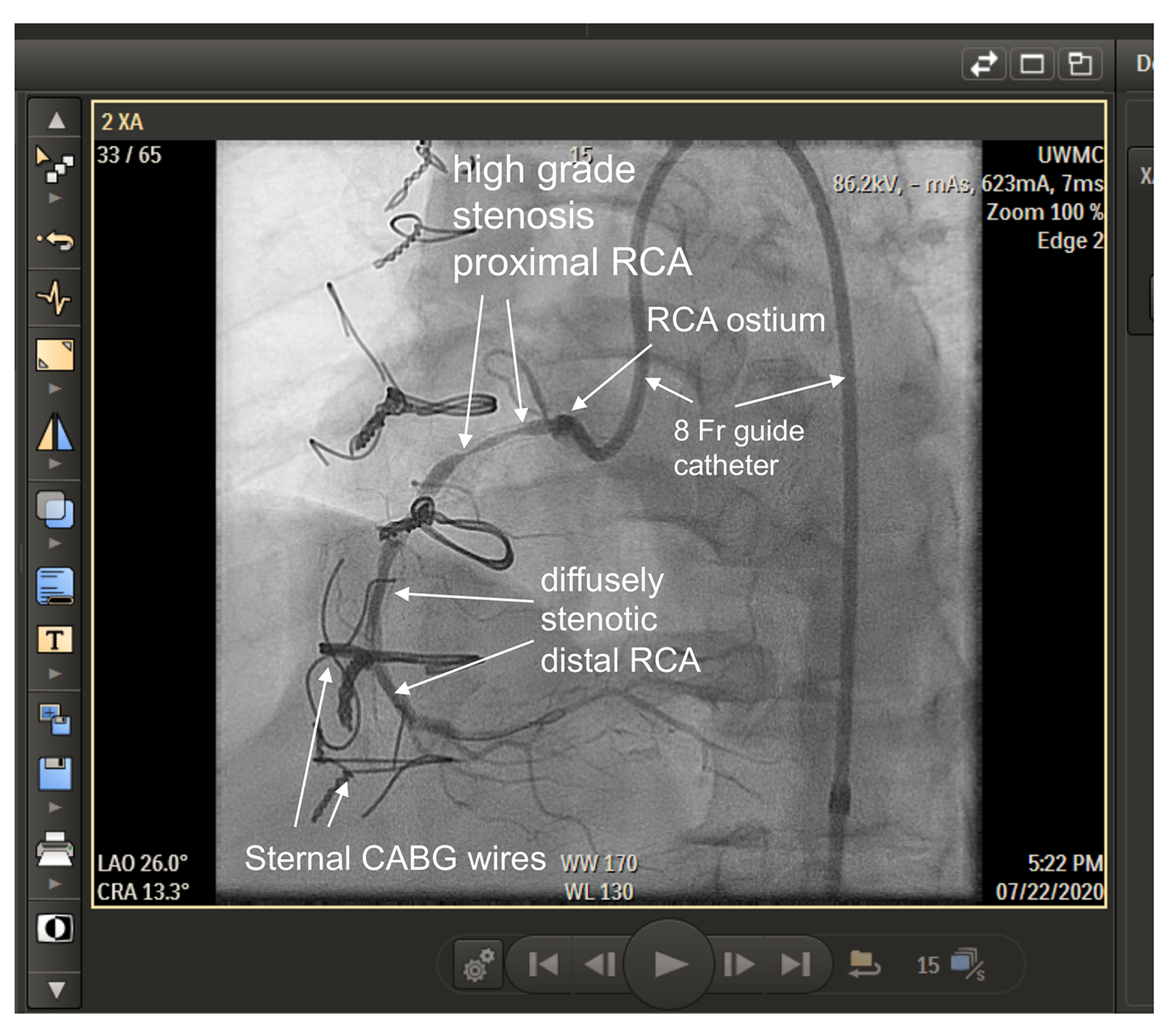
For several years, brachytherapy was the most effective treatment to prevent restenosis inside coronary stents (Figure 1). Early studies were done with iridium-192 (Ir-192), a high-energy photon emitter. Strontium-90 (Sr-90), a pure beta emitter, was later adopted to limit shielding requirements. Retrospective and prospective randomized trials showed similar outcomes with Ir-192 vs Sr-90.11,13
Drug-eluting stents (DESs) were developed in the late 1990s. In 2 randomized studies, they proved somewhat more effective than brachytherapy for treating restenosis within bare-metal stents.14,15 DES largely replaced IVBT for treatment of in-stent restenosis (ISR) after 2006. Although no study has compared IVBT for treatment of ISR specifically in DES, the convenience of stent placement led to their nearly universal adoption for ISR in DES.
First-time DESs have only about a 1% to 2% ISR rate of 1 year.15 But patients who need a second DES for same-site ISR may have a 15% to 20% chance of developing a second restenosis at 1 year.16 Factors that might account for a higher subsequent restenosis rate include drug resistance, metal hypersensitivity, stent underexpansion, barotrauma stent gap, and residual uncovered atherosclerotic plaque.17 Additionally, subsequent restenosis becomes progressively more likely as additional stents-inside-stents are placed.18,19 With an increasing chance of restenosis with more stent layers, the proper treatment for ISR of a DES has become a subject of some controversy.20 Placing additional stents in an occluded DES has been the most common solution.
IVBT is a potential alternative to angioplasty alone or inserting additional stents inside of stenosed stents. Three retrospective, uncontrolled studies suggest that IVBT may be a preferable choice for recurrent ISR, with 1-year target lesion revascularization rates of 10% to 20.21-23 These studies have led to a gradual resurgence of IVBT for patients with a second episode of ISR. IVBT was available in 45 US institutions as of 2020 (Best Vascular, email, August 29, 2022).
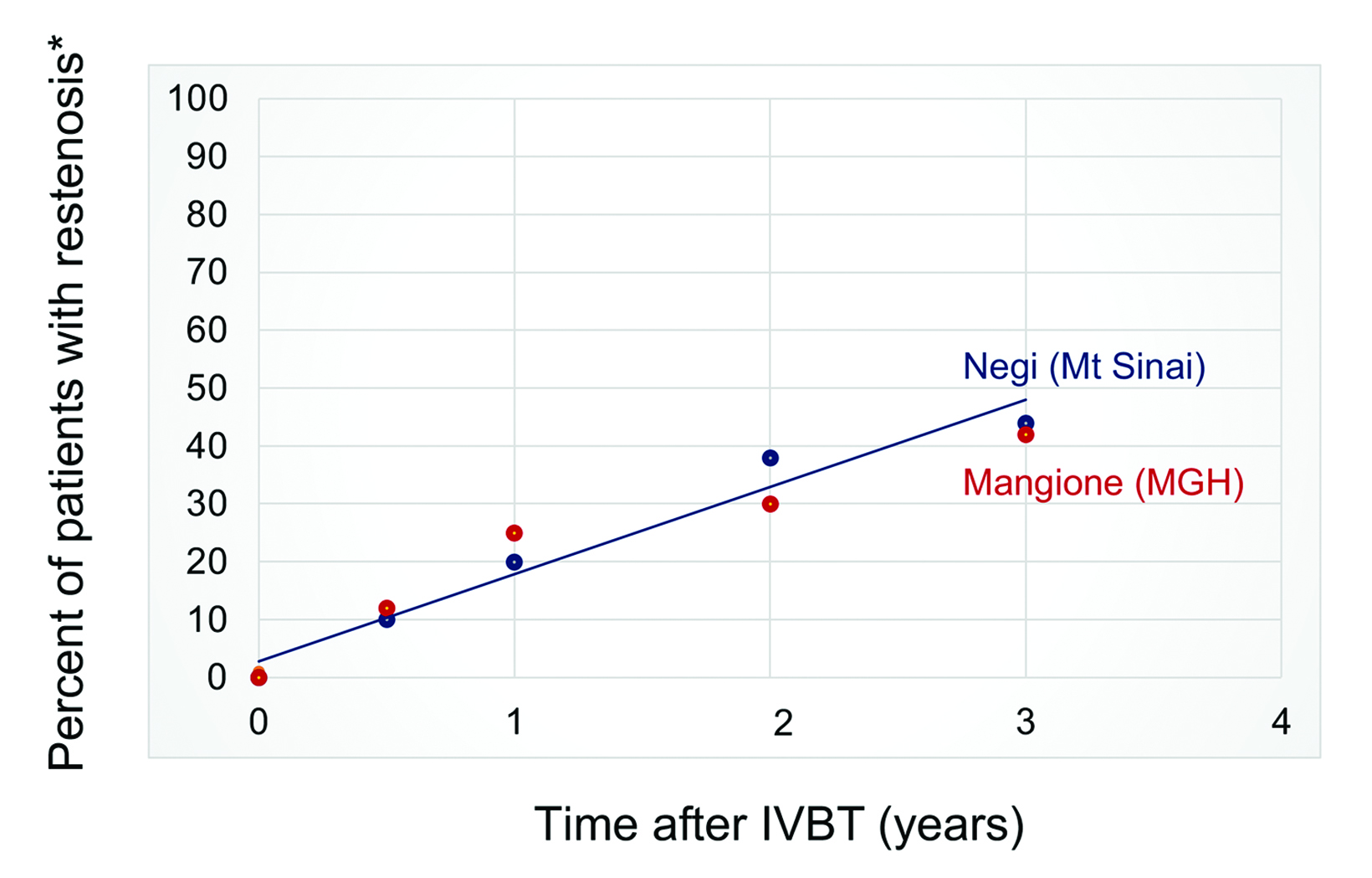
Although several observational studies suggest short-term effectiveness, the rate of late ISR after salvage IVBT remains up to 40% at 3 years, and potentially higher with longer follow-up (Figure 2).22 While moderately effective, there is clearly room to improve IVBT for multiple-recurrent ISR inside of DESs. As part of an effort to improve our institution’s program, we reviewed the published literature regarding a dose-response relationship for IVBT.
Methods
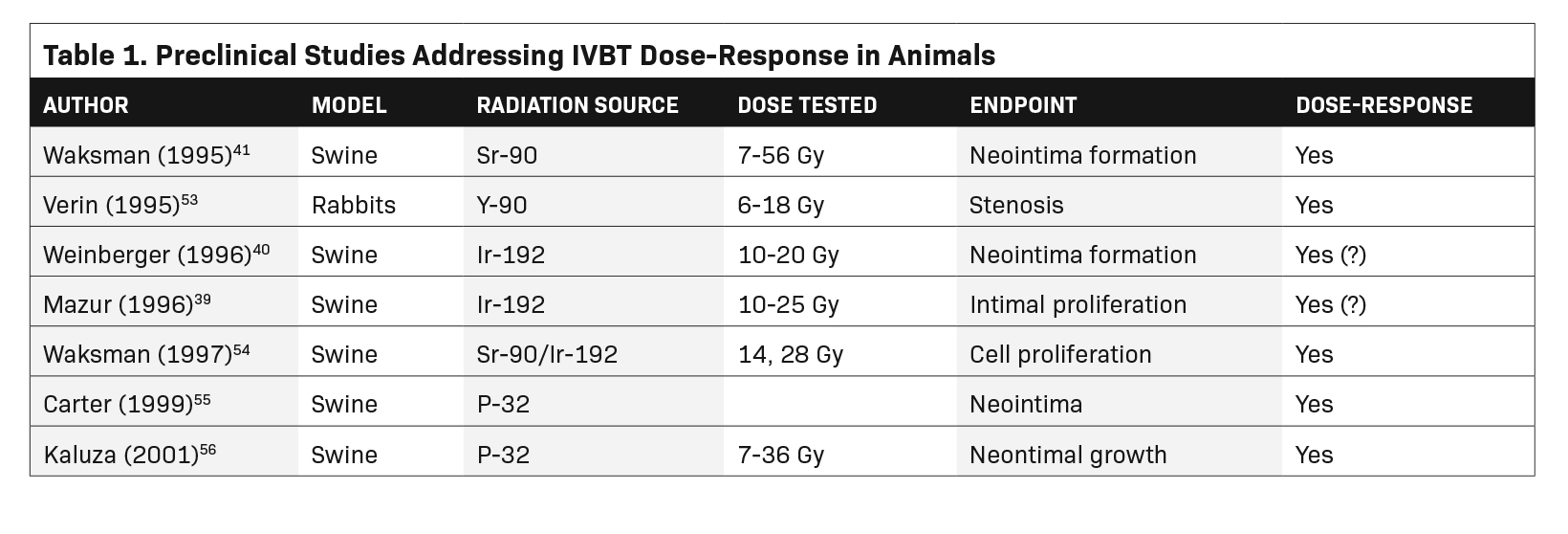
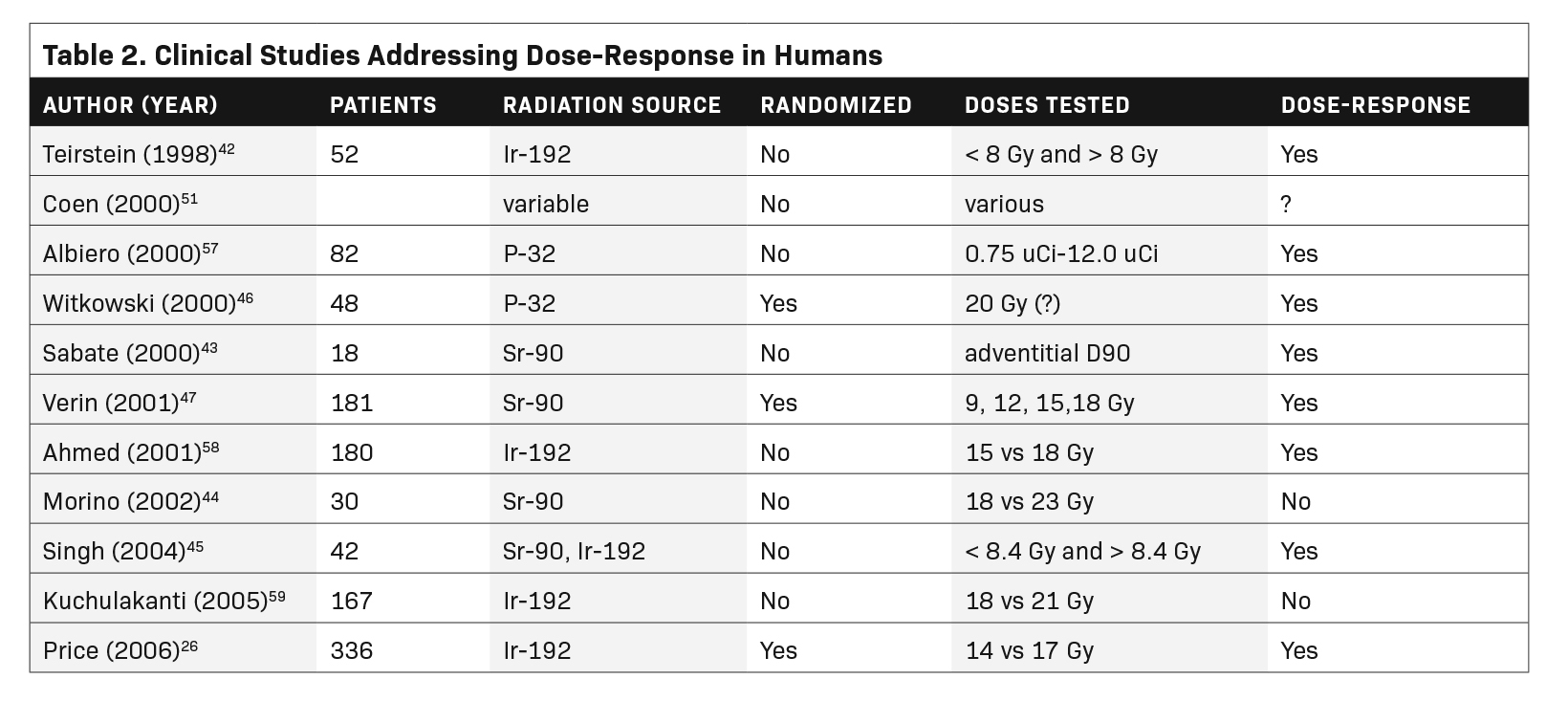
We used the PubMed search engine with the terms coronary, intravascular brachytherapy, dose, and response. The search yielded 104 articles or abstracts, 90 of which dealt with coronary brachytherapy. Of those, only 4 mentioned dose-response in the title or keywords. Starting with those, we were able to identify a total of 15 publications that considered some aspect of a dose-response relationship. The 15 studies are listed in Tables 1 and 2.
Radionuclides and Delivery Systems
Clinical studies primarily employed catheter-based temporary delivery systems, using Ir-192 or Sr-90 (Table 3). A wide variety of other isotopes and delivery systems have been considered for development, but were not extensively pursued.24 To minimize shielding requirements, the beta-emitting Sr-90 source system has been universally adopted (for now).25 Clinical studies verified comparable outcomes with gamma or beta sources.13,26
Murky Dosimetry
While there is substantial in vivo evidence of a dose-response relationship for IVBT (see below), myriad impediments remain to our understanding and manipulation of the relationship, starting with uncertainty over the intended radiation target(s), and the dose actually delivered to that target.
Smooth muscle cells (SMCs) or their progenitors are generally believed to be the effectors of restenosis.27-29 Labeling studies in pigs have shown that vascular damage (catheter-based stretching or plaque destruction) induces SMC migration from the outer to the inner vascular wall, where they generate an extracellular matrix, leading to neointimal build-up and repeat occlusion. It is generally held that radiation prevents re-occlusion by limiting SMC migration.25 The simplest mechanistic explanation is that the outer vascular wall is the radiation target, being the site of resting SMCs. However, it is unclear whether radiation’s antistenotic effect is a result of direct action on SMCs. Postradiation SMCs do not exhibit apoptosis, suggesting that direct radiation killing is not the mechanism by which radiation works.25 Instead, the anti-ISR effect may be mediated by radiation-induced cytokine release.27,30
In addition to questions about the actual radiation target, there is substantial uncertainty regarding geographic dose distribution within the vessel wall. Compared with external beam techniques, the quantification of IVBT doses is crude, complicated and undoubtedly inaccurate. By its nature, brachytherapy’s rapid dose fall-off leads to extreme heterogeneity longitudinally and circumferentially across the vessel wall, exacerbated by stents and calcium.31,32
Metal stents and calcium deposits both interfere with radiation penetration of the vessel wall.33,34 Stent lattice decreases dose transmission by approximately 20% to 30% behind the metal lattice itself.33-35 And patients commonly have had additional stents-within-stents to treat prior sites of restenosis, so the cumulative dose reduction could be far greater and complex.
Coronary calcifications also diminish dose transmission through the vessel walls. The thickness of calcium is typically highly variable along the vessel. In a heavily calcified section, the adventitial beta dose could easily be reduced twofold.33
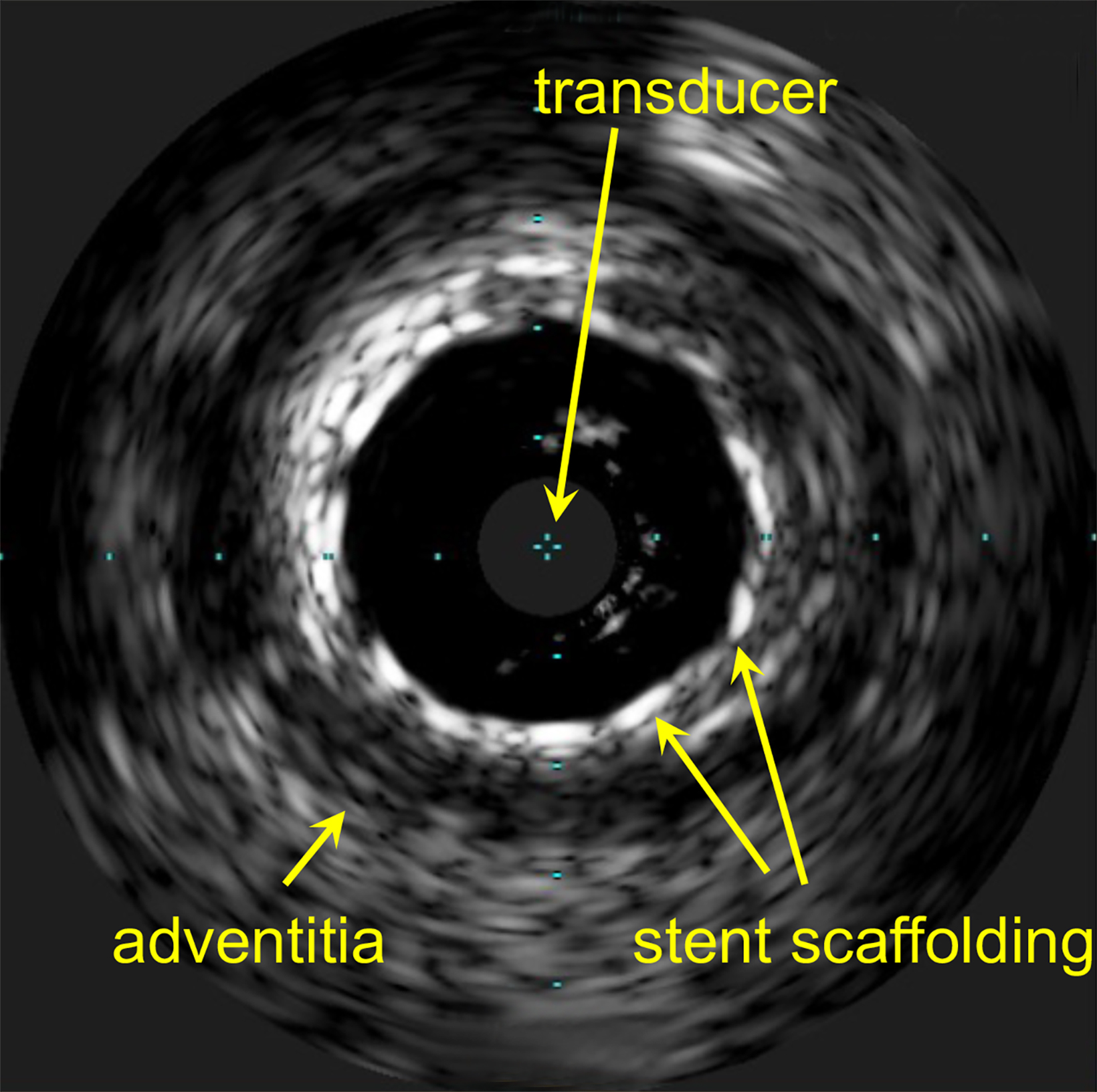
Quantifying and correcting for dose perturbations in atherosclerotic vessels is further hindered by the lack of high-quality imaging. Intravascular ultrasound (IVUS) or optical coherence tomography (OCT) are the most common modalities used. IVUS gives a fair image of vessel wall thickness in healthier vessels.36 However, metal stents and thick calcium deposits are poorly imaged, at best (Figure 3).
Geometric Obstacles
In addition to interference by stents and calcium, geometric factors substantially alter vessel wall doses. The rapid dose fall-off of Sr-90 beta leads to dramatic dose inhomogeneity in the confines of a 4-mm diameter coronary artery. There is approximately a 50% dose fall-off over 1 mm of tissue, about the thickness of a coronary vessel wall.31 Source wire asymmetry inside the vessel exacerbates fall-off by increasing dose to the near wall and decreasing dose to the far wall (Figure 4).37 Additionally, asymmetry is exacerbated by vessel curvature.32,35
Taken together, rapid dose fall-off, stents, calcium, suboptimal imaging and source asymmetry lead to great uncertainty about minimal (or maximal) doses to the vessel wall.31 Considering the doses employed over the narrow range of 10 to 25 Gy, these dose-lowering effects might significantly limit the effect of IVBT – or maybe not.
Does Dose Matter?
Where radiation is used against benign disease, a dose-response relationship is typically demonstrable. Conditions for which a relationship has been established include keloids and arteriovenous malformations.1,2 Similarly, a positive dose-response relationship has generally been shown for IVBT based on preclinical, retrospective and prospective randomized clinical trials (see below). Additionally, smooth muscle cellularity is decreased in a DES vs bare-metal stent, a phenomenon that might render DES ISR less sensitive to radiation inhibition.38 Accordingly, a dearth of SMCs might require a higher radiation dose to disrupt SMC-based restenosis.
Animal Models
There are at least 8 pre-clinical animal studies of Ir-192 or Sr-90 IVBT to prevent restenosis, 3 of which investigated a dose-response relationship.25 The results are mixed. In one of the earliest pre-clinical IVBT dose-response studies, Mazur and colleagues used a miniature swine coronary overstretch model to search for an Ir-192 dose-response for maintaining coronary patency. For such studies, the coronary vessels are damaged by overdilation with a balloon or wire stent, preceded or followed by intravascular radiation. Their results were inconclusive. Brachytherapy’s anti-stenotic effect for the left anterior descending artery increased steadily from 10 to 25 Gy at 1.5 mm from the source center. However, results were mixed for the right coronary artery (RCA) and circumflex (Cx).39
Weinberger and colleagues, also using a miniature swine model, showed increased inhibition of neointima as the dose was increased from 15 to 20 Gy (Ir-192, at the “vessel wall”). A dose of 10 Gy was associated with accelerated stenosis, a puzzling phenomenon of some concern.40 A stimulatory effect of lower doses has not been consistently reported by other investigators and may have been an artifact
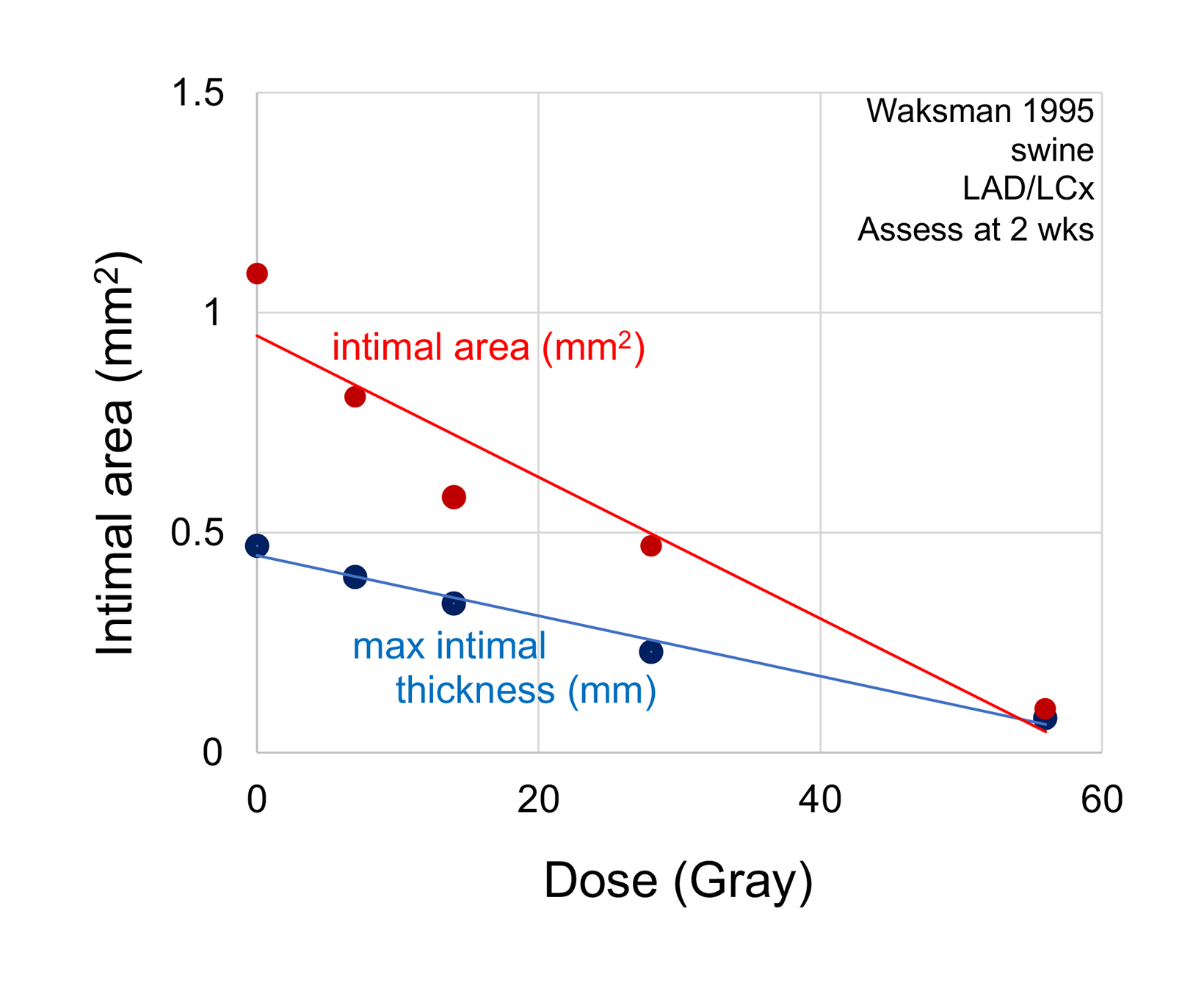
In the largest pre-clinical study, Waksman and colleagues studied 42 swine treated to doses ranging from 7 Gy to 56 Gy (Sr-90 at 2 mm from center). There was a progressive loss of maximal intimal thickness from 7 Gy to 56 Gy, with no clear limit to the anti-stenotic effect with increasing dose (Figure 5).41 There was only 1 animal in each of the 2 highest dose groups, making the statistical validity uncertain.
Retrospective Clinical Studies
Two retrospective human studies have also revealed an IVBT dose-response relationship. Teirstein and colleagues analyzed the relationship between vessel wall doses and restenosis in a prospective placebo-controlled study, using Ir-192 for ISR of bare-metal stents.42 Their nominal prescription dose was planned for 8 Gy to the most distal vessel wall, governed by a maximum dose of 30 Gy to any point on the inner vessel wall (intima). Sixteen patients received a minimal dose less than 8 Gy to the outer wall due to the 30 Gy intimal dose constraint. There was no demonstrable treatment effect in patients with a minimum vessel wall dose below 8 Gy (P = 0.72). Patients whose outer coronary wall received at least 8 Gy minimum did show a trend to a beneficial treatment effect (P = 0.081).
Sabate and colleagues looked retrospectively at vessel wall Sr-90 doses, showing lesser plaque build-up in patients with a higher adventitial dose.43 Their method of calculating the integral dose to the adventitia is not readily comparable to the point doses used by other investigators. Despite the noncomparable dose calculation methodologies and endpoints, the Teirstein and Sabate studies were consistent in suggesting a positive clinical dose-response relationship.
Other investigators have compared more sophisticated imaging-based dose metrics with revascularization success.44-46 While these efforts point to future methodology, interpretation of the studies is hampered by the poorly defined geometric and compositional complexity of the diseased vascular wall.
Prospective Clinical Studies
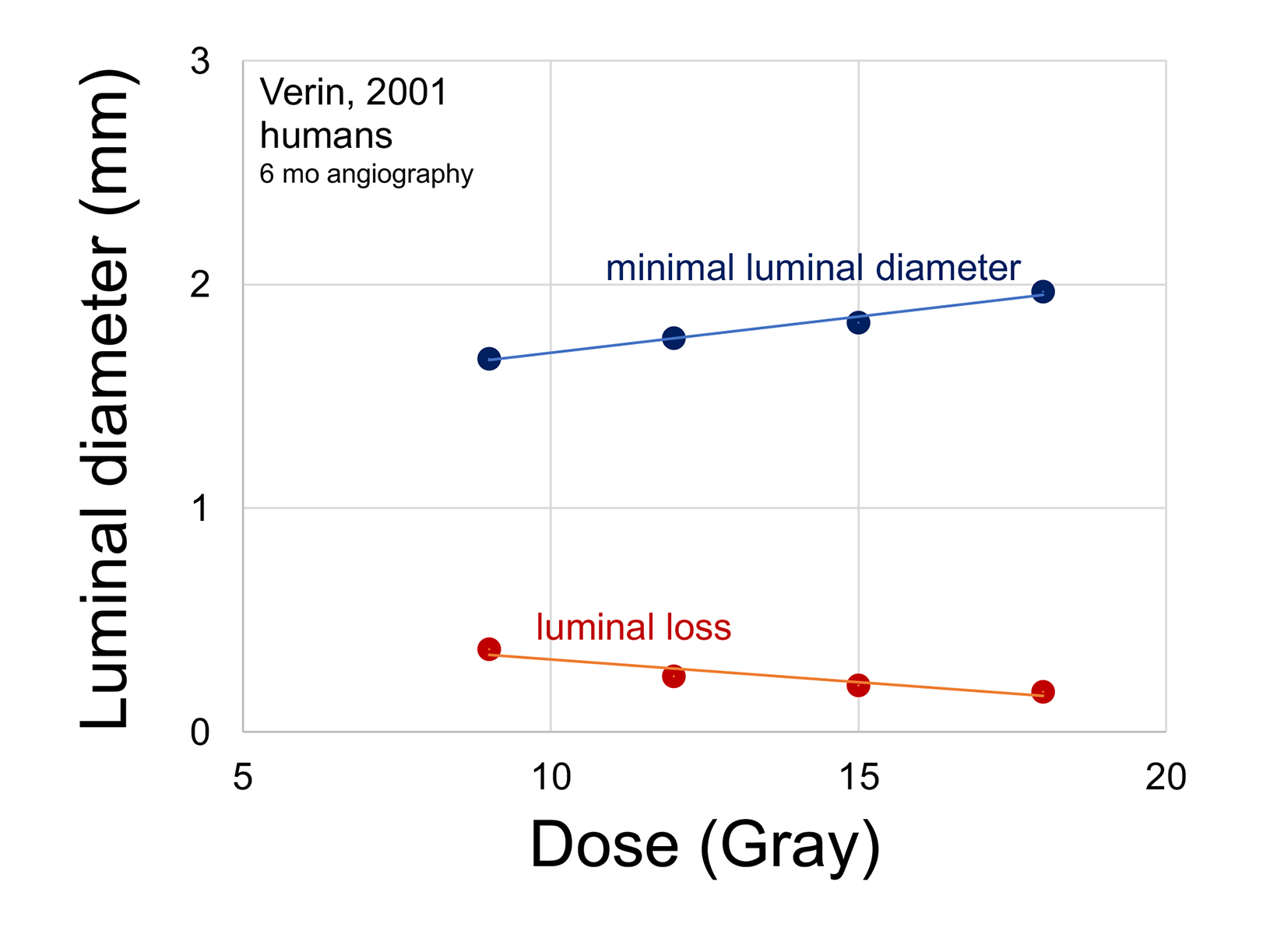
In a meticulous prospective clinical dose-response study, Verin and colleagues randomized 183 patients with de novo stenosis to Sr-90 doses of 9, 12, 15 and 18 Gy at 1 mm tissue depth. The percent of stenosis after intervention was in a narrow range of 31% to 33% among the 4 groups. Patients did not receive long-term dual antiplatelet therapy. Regardless, higher radiation doses led to progressively greater minimal luminal diameter at 6-month follow-up (Figure 6).47 Multiple angiographic indices of restenosis were increasingly improved with higher IVBT doses. There was no consistent indication of a maximal response, up to 18 Gy.
Price and colleagues randomized 336 patients to 14 vs 17 Gy (Ir-192 at 2 mm from source) for bare-metal stent ISR. The average postintervention stenosis was 37% and 35% in the 14 Gy and 17 Gy groups, respectively. Patients who received a new stent at the time of irradiation were placed on long-term dual antiplatelet therapy. At 8-month follow-up angiography, minimal luminal diameter was 1.48 for patients treated to 17 Gy vs 1.32 mm for those treated to 14 Gy (P = 0.007). In-stent stenosis as a percent of the luminal diameter decreased from 46% to 37% with the higher radiation dose (P = 0.009). Overall adverse cardiac events decreased from 28% to 17% in patients who received the higher dose (P = 0.018).26
Like the retrospective studies, the 2 prospective randomized clinical studies are not readily comparable, as they used different source types (Sr-90 vs Ir-192), dose specification (1 vs 2 mm from center) and endpoints. Nonetheless, they both showed a consistent relationship between higher prescription dose and greater effectiveness at preventing restenosis. Not known is whether prescription doses higher than the currently used 18 to 23 Gy are more effective (and safe). Had the popularity of IVBT not plummeted abruptly with the introduction of DES, the upper limit of the dose-response would likely have been studied years ago.
Does Dosimetry Matter?
It is hard to reconcile the extreme technical challenges of IVBT dosimetry with the surprisingly consistent clinical evidence of effectiveness and of a dose-response relationship. Even though we cannot quantify the dose well, the procedure works, and there is a dose-response relationship. Perhaps precise dosimetry is not necessary.
In planning radiation therapy treatments, great effort is made to achieve dose uniformity, with the intention of delivering a minimal tumoricidal dose to all potential sites of malignant cells. If the antistenotic effect of IVBT were analogous to cancer eradication, dose perturbations behind metal stent lattice and calcium deposits would severely limit its effectiveness. But dosimetric goals may be different for vascular brachytherapy. If cytokine perturbations rather than direct SMC killing are the mechanism of radiation-suppressed restenosis, dose heterogeneity may not be so important.
The generation of effector cytokines in higher-dosed parts of the vessel wall would presumably not be substantially compromised by dose heterogeneity. And cytokine diffusion within the vessel wall could minimize the effect of radiation dose inhomogeneity resulting from stents, calcium and source asymmetry. In other words, underdosed areas in the vessel wall would not limit radiation effectiveness in the way that underdosed regions can spare cancer cells. This could explain the consistent effectiveness and dose responsiveness of IVBT despite the heterogeneous, unpredictable dosimetry. It would also still allow for a dose-response relationship, despite unpredictable dosimetry, and would be a rationale for increasing the current prescription dose(s).
Are Higher Prescription Doses Safe?
Despite early animal studies predicting late radiation injury, remarkably little clinically evident toxicity has been reported after IVBT.48 There were early reports of excessive late thrombosis after IVBT, leading to longer use of dual antiplatelet therapy (DAPT). DAPT may decrease the late thrombosis rate, at least in patients who have additional stents placed at the time of IVBT.49,50 In current practice, placement of additional stents at the time of IVBT is not routine, but use of prolonged DAPT is commonly recommended.
Candado and colleagues raised some early concern about vessel tolerance when they reported a pseudoaneurysm in a series of 19 patients.6 It was not clear if the aneurysm was related to radiation or the angioplasty procedure. Regardless, no reports of excessive or unusual radiation-related complications have emerged, despite many thousands of patients being treated on and off trials. Nor have reports from multiple series suggested a higher incidence of major cardiac events in IVBT patients compared with those treated with angioplasty or repeat stenting. With higher doses used in some series, radiation-related complications have not emerged. Coen and colleagues treated 28 patients with 28 to 42 Gy prescription doses (P-32 at 2 mm from the source center), substantially higher than the current 18 to 23 Gy typically prescribed with Sr-90. They did not report excess toxicity.51
More reassuring of the safety of higher doses is a re-treatment series published by Waksman and colleagues.52 They retreated 51 patients with a second IVBT, 6 months or more after an initial IVBT, with no apparent complications. Even a doubling of dose by adding a second treatment seems to not be accompanied by complications, again suggesting that doses well above the current maximum of 18 to 23 Gy are relatively safe. Considering the lack of reported complications despite meticulous follow-up of hundreds of patients enrolled in studies, it seems likely that coronary vessel tolerance to radiation is higher than the current 18 to 23 Gy. Just how much higher the prescription doses can safely go to is a matter of conjecture, considering the lack of radiation-related complications to date.
Conclusion – Where to From Here?
Despite dosimetric uncertainties, coronary brachytherapy has repeatedly proven effective, decreasing restenosis by half.7 This effectiveness comes despite the unavoidable dose uncertainties and inhomogeneities with current technology. But considering the 40% recurrence rate at 3 years, there is clearly room for improvement. Better methods to tailor dose delivery to individual patients’ vessel walls seem possible, and may offer increased effectiveness.35
In summary, dose-response studies mostly point to increasing effect with increasing dose. Prior investigators have not found an upper limit to the dose-response relationship, and there have been remarkably few complications at current doses. Despite far-from-perfect dosimetry, it seems that the most logical, simple way to increase IVBT effectiveness is by increasing the prescription dose or prescription depth, even using the crude system we have now. Given the high rate of target lesion failure in patients with recalcitrant, recurrent in-stent restenosis, the paucity of options for such patients, and the modest benefit of current brachytherapy protocols, further clinical studies with higher prescription doses seem warranted. The authors choose not to speculate specifically at this time as to how high the doses should be raised. However, a substantial increase would seem justifiable, given the lack of clinically evident complications with current doses.
References
- Flickinger JC, Pollock BE, Kondziolka D, Lunsford LD. A dose-response analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36(4):873-879.
- Kal HB, Veen RE, Jurgenliemk-Schulz IM. Dose-effect relationships for recurrence of keloid and pterygium after surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2009;74(1):245-251.
- de Haan A, van Nes JGH, Werker PMN, Langendijk JA, Steenbakkers R. Radiotherapy for patients with Ledderhose disease: long-term effects, side effects and patient-rated outcome. Radiother Oncol. 2022;168:83-88.
- Popovic M, Agarwal A, Zhang L, et al. Radiotherapy for the prophylaxis of heterotopic ossification: a systematic review and meta-analysis of published data.2014;113(1):10-17.
- Friedman M, Felton L, Byers S. The antiatherogenic effect of iridium-192 upon the cholesterol-fed rabbit. J Clin Invest. 1964;43:185-192.
- Condado JA, Waksman R, Gurdiel O, et al. Long-term angiographic and clinical outcome after percutaneous transluminal coronary angioplasty and intracoronary radiation therapy in humans. Circulation. 1997;96(3):727-732.
- Bhargava B, Tripuraneni P. Role of intracoronary brachytherapy for in-stent restenosis? Lancet. 2002;359(9306):543-544.
- Teirstein PS, Massullo V, Jani S, et al. Catheter-based radiotherapy to inhibit restenosis after coronary stenting. N Engl J Med. 1997;336(24):1697-1703.
- Leon MB, Teirstein P, Moses JW. Localized intracoronary gamma-radiation therapy to inhibit the recurrence of restenosis after stenting. N Engl J Med. 2001;344:250-256.
- Waksman R, White LR. intracoronary gamma radiation therapy after angioplasty inhibits recurrence in patients with in-stent restenosis. Circulation. 2000;101:2165-2171.
- Popma JJ, Suntharalingam M, Lansky AJ, et al. Randomized trial of 90Sr/90Y beta-radiation versus placebo control for treatment of in-stent restenosis. Circulation. 2002;106(9):1090-1096.
- Waksman R, Raizner AE, Yeung AC, Lansky A. Use of localised intracoronary beta radiatoion in treatment of in-stent restenosis: the INHIBIT randomised controlled trial. lancet. 2002;359:551-557.
- Shirai K, Lansky AJ, Mintz GS, et al. Comparison of the angiographic outcomes after beta versus gamma vascular brachytherapy for treatment of in-stent restenosis. Am J Cardiol. 2003;92(12):1409-1413.
- Holmes DR, Jr., Teirstein P, Satler L, et al. Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial. JAMA. 2006;295(11):1264-1273.
- Stone GW, Ellis SG, O’Shaughnessy CD, et al. Paclitaxel-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the TAXUS V ISR randomized trial. JAMA. 2006;295(11):1253-1263.
- Mehilli J, Byrne RA, Tiroch K, et al. Randomized trial of paclitaxel-versus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents: the ISAR-DESIRE 2 (Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2) study. J Am Coll Cardiol. 2010;55(24):2710-2716.
- Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56(23):1897-1907.
- Raber L, Juni P, Loffel L, et al. Impact of stent overlap on angiographic and long-term clinical outcome in patients undergoing drug-eluting stent implantation. J Am Coll Cardiol. 2010;55(12):1178-1188.
- Cheneau E, Wu Z, Leborgne L, et al. Additional stenting promotes intimal proliferation and compromises the results of intravascular radiation therapy: an intravascular ultrasound study. Am Heart J. 2003;146(1):142-145.
- Siontis GC, Stefanini GG, Mavridis D, et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. 2015;386(9994):655-664.
- Negi SI, Torguson R, Gai J, et al. Intracoronary brachytherapy for recurrent drug-eluting stent failure. Cardiovasc Interv. 2016;9(12):1259-1265.
- Mangione FM, Jatene T, Badr Eslam R, et al. Usefulness of intracoronary brachytherapy for patients with resistant drug-eluting stent restenosis. Am J Cardiol. 2017;120(3):369-373.
- Varghese MJ, Bhatheja S, Baber U, et al. Intravascular brachytherapy for the management of repeated multimetal-layered drug-eluting coronary stent restenosis. Circ Cardiovasc Interv. 2018;11(10):e006832.
- Chiu-Tsao ST, Schaart DR, Soares CG, Nath R, No ATPCTG. Dose calculation formalisms and consensus dosimetry parameters for intravascular brachytherapy dosimetry: recommendations of the AAPM Therapy Physics Committee Task Group No. 149. Med Phys. 2007;34(11):4126-4157.
- Sims EC, Rothman MT, Warner TD, Powell ME. Coronary artery brachytherapy. Clin Oncol. 2002;14(4):313-326.
- Price MJ, Moses JW, Leon MB, et al. A multicenter, randomized, dose-finding study of gamma intracoronary radiation therapy to inhibit recurrent restenosis after stenting. J Invasive Cardiol. 2006;18(4):169-173.
- Rubin P, Williams JP, Riggs PN, et al. Cellular and molecular mechanisms of radiation inhibition of restenosis. Part I: role of the macrophage and platelet-derived growth factor. Int J Radiat Oncol Biol Phys. 1998;40(4):929-941.
- Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992;86(3):723-729.
- Wilcox JN, Waksman R, King SB, Scott NA. The role of the adventitia in the arterial response to angioplasty: the effect of intravascular radiation. Int J Radiat Oncol Biol Phys. 1996;36(4):789-796.
- Scott NA. Restenosis following implantation of bare metal coronary stents: pathophysiology and pathways involved in the vascular response to injury. Adv Drug Deliv Rev. 2006;58(3):358-376.
- DeCunha J, Janicki C, Enger SA. A retrospective analysis of catheter-based sources in intravascular brachytherapy. Brachytherapy. 2017;16(3):586-596.
- DeCunha JM, Enger SA. A new delivery system to resolve dosimetric issues in intravascular brachytherapy. Brachytherapy. 2018;17(3):634-643.
- Li XA, Wang R, Yu C, Suntharalingam M. Beta versus gamma for catheter-based intravascular brachytherapy: dosimetric perspectives in the presence of metallic stents and calcified plaques. Int J Radiat Oncol Biol Phys. 2000;46(4):1043-1049.
- Fan P, Chiu-Tsao ST, Patel NS, et al. Effect of stent on radiation dosimetry in an in-stent restenosis model. Cardiovasc Radiat Med. 2000;2(1):18-25.
- Li XA, O’Neill M, Suntharalingam M. Improving patient-specific dosimetry for intravascular brachytherapy. Brachytherapy. 2005;4(4):291-297.
- Carlier SG, Coen VL, Sabate M, et al. The role of intravascular ultrasound imaging in vascular brachytherapy. Int J Cardiovasc Intervent. 2000;3(1):3-12.
- Sehgal V, Li Z, Palta JR, Bolch WE. Dosimetric effect of source centering and residual plaque for beta-emitting catheter based intravascular brachytherapy sources. Med Phys. 2001;28(10):2162-2171.
- Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J. 2015;36(47):3320-3331.
- Mazur W, Ali MN, Khan MM, et al. High dose rate intracoronary radiation for inhibition of neointimal formation in the stented and balloon-injured porcine models of restenosis: angiographic, morphometric, and histopathologic analyses. Int J Radiat Oncol Biol Phys. 1996;36(4):777-788.
- Weinberger J, Amols H, Ennis RD, Schwartz A, Wiedermann JG, Marboe C. Intracoronary irradiation: dose response for the prevention of restenosis in swine. Int J Radiat Oncol Biol Phys. 1996;36(4):767-775.
- Waksman R, Robinson KA, Crocker IR, et al. Intracoronary low-dose beta-irradiation inhibits neointima formation after coronary artery balloon injury in the swine restenosis model. Circulation. 1995;92(10):3025-3031.
- Teirstein PS, Massullo V, Jani S, et al. A subgroup analysis of the Scripps Coronary Radiation to Inhibit Proliferation Poststenting Trial. Int J Radiat Oncol Biol Phys. 1998;42(5):1097-1104.
- Sabate M, Marijnissen JP, Carlier SG, Kay IP, van der Giessen WJ, Coen VL, et al. Residual plaque burden, delivered dose, and tissue composition predict 6-month outcome after balloon angioplasty and beta-radiation therapy. Circulation. 2000;101(21):2472-2477.
- Morino Y, Kaneda H, Fox T, et al. Delivered dose and vascular response after beta-radiation for in-stent restenosis: retrospective dosimetry and volumetric intravascular ultrasound analysis. Circulation. 2002;106(18):2334-2339.
- Singh HS, Yue N, Azimi N, Nath R, Roberts KB, Pfau S. Relation of clinical success in coronary brachytherapy to dose. Am J Cardiol. 2004;94(7):847-852.
- Witkowski A, Chmielak Z, Kalinczuk L, et al. Optimization of dose prescription protocol and its impact on delivered dose and vascular response after beta-radiation for in-stent restenosis. A randomized trial with serial volumetric intravascular ultrasound and dose volume histograms. Cardiovasc Revasc Med. 2006;7(1):34-40.
- Verin V, Popowski Y, de Bruyne B, et al. Endoluminal beta-radiation therapy for the prevention of coronary restenosis after balloon angioplasty. The Dose-Finding Study Group. N Engl J Med. 2001;344(4):243-249.
- Powers BE, Thames HD, Gillette EL. Long-term adverse effects of radiation inhibition of restenosis: radiation injury to the aorta and branch arteries in a canine model. Int J Radiat Oncol Biol Phys. 1999;45(3):753-759.
- Costa MA, Sabate M, van der Giessen WJ, et al. Late coronary occlusion after intracoronary brachytherapy. Circulation. 1999;100(8):789-792.
- Waksman R, Ajani AE, White RL, et al. Two-year follow-up after beta and gamma intracoronary radiation therapy for patients with diffuse in-stent restenosis. Am J Cardiol. 2001;88(4):425-428.
- Coen VL, Knook AH, Wardeh AJ, et al. Endovascular brachytherapy in coronary arteries: the Rotterdam experience. Cardiovasc Radiat Med. 2000;2(1):42-50.
- Waksman R, Lew R, Ajani AE, et al. Repeat intracoronary radiation for recurrent in-stent restenosis in patients who failed intracoronary radiation. Circulation. 2003;108(6):654-656.
- Verin V, Popowski Y, Urban P, et al. Intra-arterial beta irradiation prevents neointimal hyperplasia in a hypercholesterolemic rabbit restenosis model. Circulation. 1995;92(8):2284-2290.
- Waksman R, Rodriguez JC, Robinson KA, et al. Effect of intravascular irradiation on cell proliferation, apoptosis, and vascular remodeling after balloon over-stretch injury of porcine coronary arteries. Circulation. 1997;96(6):1944-1952.
- Carter AJ, Scott D, Bailey L. Dose-response effects of 32P radioactive stents in an atherosclerotic porcine coronary model. Circulation. 1999;100(14):1548-1554.
- Kaluza GL, Raizner AE, Mazur W, et al. Dose-response study of intracoronary beta-radiation with 32P in balloon- and stent-injured coronary arteries in swine. Cardiovasc Radiat Med. 2001;2(4):225-230.
- Albiero R, Adamian M, Kobayashi N, et al. Short- and intermediate-term results of (32)P radioactive beta-emitting stent implantation in patients with coronary artery disease: The Milan Dose-Response Study. Circulation. 2000;101(1):18-26.
- Ahmed JM, Mintz GS, Waksman R, et al. Serial intravascular ultrasound assessment of the efficacy of intracoronary gamma-radiation therapy for preventing recurrence in very long, diffuse, in-stent restenosis lesions. Circulation. 2001;104(8):856-859.
- Kuchulakanti P, Torguson R, Canos D, et al. Optimizing dosimetry with high-dose intracoronary gamma radiation (21 Gy) for patients with diffuse in-stent restenosis. Cardiovasc Revasc Med. 2005;6(3):108-112.
Citation
K W, M K, KE K, RS H, K N, ZL S, JM M, WL L, ML P, C D. The Evidence and Rationale for a Coronary Brachytherapy Dose-Response. Appl Radiat Oncol. 2022;(3):18-26.
October 14, 2022