The global radiation oncology workforce in 2030: Estimating physician training needs and proposing solutions to scale up capacit
Images


SA-CME credits are available for this article here.
Radiation therapy access is insufficient in low- and middle-income countries.1 As cancer cases are projected to increase in countries of all economic tiers, the need for radiation therapy will continue to expand.2 Several analyses have focused on radiation therapy equipment needs as inferred by national cancer burdens.3,4 However, radiation therapy services depend on factors beyond equipment, with factors such as quality and safety management and human resources playing an important role.5,6 Human resources, and well-trained radiation and clinical oncologists in particular, are essential to ensuring access, efficacy, quality and safety of radiation therapy. This review will explore the capacity gap in radiation therapy through the lens of human resource needs. We will model the current and projected radiation oncologist training needs and investigate the disparity between high-income countries vs low- and middle-income countries. We will then examine existing and novel solutions to radiation oncologist physician training and licensure. We will focus on the critical nature of regional collaboration between countries in different income strata to meet physician training needs for low-income countries.
Methods
Cancer Incidence and Income Groups
The International Agency for Research on Cancer (IARC) recently updated the Global Cancer Incidence, Mortality and Prevalence database (GLOBOCAN), providing revised estimates of cancer incidence and mortality in September 2018.7 Using the accompanying web-based platform, the Global Cancer Observatory (GCO) cancer burden estimates for the 173 countries analyzed by the Global Task Force on Radiotherapy for Cancer Control (GTF RCC) were obtained for 2018 and 2030.1,2 The individual country datasets were then grouped according to the World Bank income groups classification for 2017 into high-income (50 countries), upper-middle-income (46 countries), lower-middle-income (47 countries), and low-income (30 countries).8
Equipment Needs and Costs
The evidence-based estimation (EBEST) method from the Collaboration for Cancer Outcomes Research and Evaluation (CCORE) was used to calculate the number of radiation therapy courses required in 2018 and 2030 based on the cancer incidence for each income group.9-11 The required number of investment, machines and staff to deliver these courses was then calculated using the activity-based costing model used by the GTF RCC.1 Because the EBEST method has the potential to overestimate actual needs if radiation therapy utilization rates are not optimized, we also included a “lower estimate” using published Criterion-Based Benchmarking (CBB) estimates of radiation therapy utilization rates within 1 year of diagnosis (RT 1Y). We used 26% RT1Y for our “lower estimate” based on recently published Australian data by Barton et al, which was 7% lower than the rate reported by Mackillop et al for Canada.12,13
Available Machines, Staffing Level and Training Program Capacity
The number of radiation therapy centers and megavoltage machines in 2018 was obtained from the IAEA Directory of Radiotherapy Centres (DIRAC).14 Due to the lack of reliable data on the availability of radiation oncologists on a global level, we estimated the number of radiation oncologists needed to deliver optimal radiation therapy services with the number of existing machines for each income group using the approach and assumptions used by the GTF RCC.1 The number of radiation oncologist full-time equivalents (FTEs) obtained from this calculation was used to represent the current number of practicing radiation oncologist FTEs in 2018. Using the same model, the projected number of radiation therapy courses in 2030 was used to calculate the required number of megavoltage machines and practicing radiation oncologist FTEs for 2030.
Training
There are several recommendations regarding the maximum number of residency positions in a training program. The European Society for Radiation Therapy and Oncology (ESTRO) recommended that the number of residents in a training program should not exceed the number of FTE staff.15,16 The Accreditation Council for Graduate Medical Education (ACGME) required at least four FTE radiation oncologists at the primary clinical site dedicated to teaching activities with at least a 0.67 FTE faculty-to-resident ratio.17 Both ratios were used to estimate training program capacity based on the number of practicing radiation oncologist FTEs for each income group. The proportion of practicing radiation oncologists involved in training residents was adjusted iteratively until the number available in 2030 matched the projected needs, or 100% involvement was reached (maximum potential capacity).
Most published curricula for radiation oncology residency training required 5 years from entry to certification, with some variation in terms of entry points to the training program following completion of medical school.18,19 The IAEA Syllabus for the Education and Training of Radiation Oncologists, published in 2009, recommended at least 3 years of residency training.20 Both scenarios were considered in a sensitivity analysis.
Results
Equipment Needs in 2030
There were 7100 radiation therapy centers worldwide in 2018, 66% of which were in high-income countries (Figure 1). The number of megavoltage machines in high-income countries were 8444, and combined with the 3870 megavoltage machines in upper-middle-income countries they constitute 98% of the world’s megavoltage machines, leaving the remaining 2% in lower-middle- and low-income countries—212 and 26 machines, respectively.
In 2030, there will be a 21% and 32% increase in the projected cancer incidence in high-income countries and upper-middle-income countries, respectively, compared to 2018. Lower-middle- and low-income countries will see even higher rates of increase of 38% and 34%, respectively. Under the same set of assumptions as in the GTF RCC publication, these increases in cancer incidence will raise the required number of machines in 2030 to 9716 machines in high-income countries, 7872 in upper-middle-income countries, 4134 in low- and middle-income countries, and 610 in low-income countries, corresponding to a total investment of USD 82.7 billion in capital and USD 31.7 billion in training.
Human Resources Supply and Demand
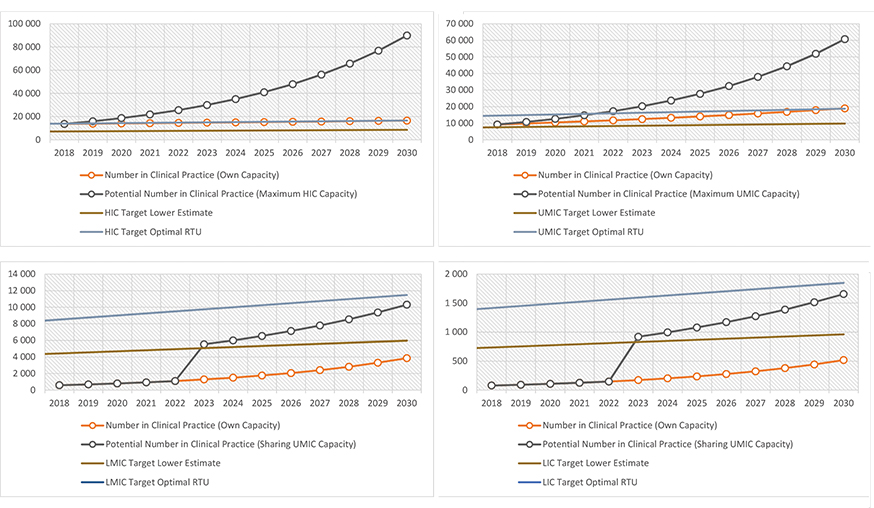
Assuming there are currently enough radiation oncologists to provide resource-optimized care with the existing number of machines in 2018, we estimated 664 practicing radiation oncologists in lower-middle- and low-income countries. This number needs to grow to 13 322 over the next 12 years to provide optimal radiation therapy access by 2030, assuming enough investment is made in infrastructure. Currently, this would require an increase at the rate of 28% annually without considering any loss from the pool of practicing radiation oncologists including retirement, which, assuming a 30-year interval between training completion and retirement, occurs at a rate of 3% per year.
Even if we assume that every radiation oncology center in low- and low-middle-income countries merge to create one common training program using a 5-year common curriculum, with every practicing radiation oncologist involved as teaching faculty at the recommended ratio of 1 FTE staff per resident (100% involvement), only a net 17% growth could be sustained annually. The deficit of radiation oncologist FTEs remains constant at 8900 despite the increase from 664 in 2018 to 4371 in 2030. (Figure 2 C,D)
Reducing the FTE requirement can potentially increase the capacity of training programs to the level required to achieve at least the “lower estimate” of the needs in 2030, and so can reduction in the length of training. Shortening the training duration to 4 years enables the projected number to reach the “lower estimate” number of radiation oncologists, while reducing it further to 3 years or using a staff/resident ratio of 2:3 (0.67 FTE staff per resident) both dramatically boost capacity. (Figure 3)
A significant proportion of the radiation oncology workforce FTEs in upper-middle-income countries will need to be involved as teaching faculty to provide enough capacity for training programs to increase practicing radiation oncologists from an estimated 9228 in 2018 to 18 797 in 2030. Assuming the same 5-year curriculum and FTE requirement for residency training, 46% of practicing radiation oncologists in upper-middle-income countries would need to be involved in residency training (Figure 2B). Assuming the “excess” capacity of 54% is utilized to train radiation oncologists for low- and low-middle-income countries, it appears possible to reach the “lower estimate” number of radiation oncologists in low- and low-middle-income countries although the total number still falls short of the needs estimated by optimal RTUs (Figure 2C,D).
High-income countries, on the other hand, would be more concerned about fine-tuning program requirements to prevent oversupply of radiation oncologists, because only a small proportion (23%) of practicing radiation oncologist FTEs need to be involved in a residency program to increase practicing radiation oncologists from 13 665 in 2018 to 16 575 in 2030 (Figure 2A).
Cost Considerations
The estimated training costs of such an endeavor at scale are quite significant. The GTF RCC estimated full training costs per trainee of USD 550 000 for high-income countries and USD 100 000 for upper-middle, low-middle, and low-income countries.1 With the training costs in high-income countries more than 5 times as expensive, there is a strong cost/logistics rationale for prioritizing training support from upper-middle-income countries. However, if only upper-middle income countries were involved in training support, there would still be a shortage of an estimated 1361 radiation oncologists by 2030, assuming optimal utilization rates.
Discussion
Scaling Up Training in Low- and Low-Middle-Income Countries
We found that even when we used optimistic assumptions on a simple model, grossly overestimating the growth of radiation oncologist supply, it was still extremely difficult for low- and low-middle-income countries to train enough professionals to keep up with the optimal infrastructure investment needed by 2030. The training capacity in these countries would limit the potential rate of growth in radiation therapy access to no more than 17% annually, regardless of investments in infrastructure. In reality, the number that could be trained would be much lower because in most countries only practicing radiation oncologists working in the few accredited radiation oncology programs would be involved in training residents. High-income countries, on the other hand, tend to have a higher capacity than expected demand, necessitating in some cases regulation to avoid oversupply.21 This is not true for all high-income countries, however, with countries such as the UK facing a shortage of clinical oncologists that is expected to worsen in the next 5 years unless training capacity doubles and work conditions improve.22 Canada has also noted an incremental increase in supply of radiation oncologists with rising caseloads, potentially suggesting an increased training need.23
Unless drastic changes are implemented, it is likely that the radiation oncologist deficit will continue to widen if lower-middle- and low-income countries are tasked with training their own radiation oncologists. Compromises in length of training or FTE requirements could potentially accelerate the growth, but these will have to be carefully planned to avoid a negative impact on quality and safety. High- and upper-middle-income countries can potentially help offset the low supply in lower-middle- and low-income countries, and such efforts are ongoing on a small scale, with residency programs hosting a few international trainees per year. However, mobilizing and financing the residency training at scale would be a significant undertaking. Besides the costs and logistics involved, there is a potential risk of migration that could further exacerbate the capacity mismatch between lower- and higher-income nations. Regional collaborations would need to be established so that excess capacity from upper-middle- and high-income countries can be optimally utilized by their neighboring low- and low-middle-income countries to the maximum possible extent while keeping the risk of loss due to migration to a minimum.
Harmonization
For the collaboration to succeed, mutual understanding and shared vision will be necessary. Many components of the residency training curriculum will need to be harmonized to establish the degree of expertise required for a concerted regional or global effort in training future radiation oncologists.
The IAEA recognized the need for harmonization and prepared a syllabus to guide managers and directors of radiation oncology training programs in establishing or upgrading a training program for radiation oncologists.20 The syllabus, published in 2010 and endorsed by major professional societies, was designed to be implementable within the various limitations in available resources while maintaining a high educational standard. Considering that more than 10 years have passed since it was drafted, however, the syllabus will need to be updated to keep up with recent developments and best practices in the field of radiation oncology, incorporating best practices in postgraduate medical education while remaining resource-aware and system-neutral. Of particular interest would be the potential for a modular, flexible-length training program incorporating a competency-based curriculum, which would enable training duration to be adjusted to the level of needs. The ACGME has begun to pilot such a system in several residency training programs, although radiation oncology is not among these.24
The existence of a harmonized curriculum can potentially facilitate the mobility of trainees and teaching staff, allowing expertise to flow freely within the region but at the same time increasing the possibility of permanent emigration. In a recent survey conducted by ESTRO, 77% of trainees expressed some interest in working in a different country than where they were trained.15 Similarly, in 2016, the World Bank recognized that higher education was an important avenue for facilitating the emigration of high-skilled workers.25
Left to the market mechanism of supply and demand, emigration can worsen the disparity between low- and high-income countries in access to trained radiation oncologists. A control and incentive mechanisms would be needed to prevent outflow of skills and expertise from lower-middle- and low-income countries. In other specialty training programs, this has included a minimum service commitment to the physician’s country or region of origin.26,27 Alternatively, incentives have been provided in areas that may be less sought after to recruit and retain qualified health practitioners.28 However, despite the disadvantages of migration, accreditation standards must be shared or mutually recognized across training regions or partnerships.
International Collaborations
When properly coordinated and maintained, shared learning resources will allow efficient use of available resources by reducing the teaching workload of faculty in training programs, allowing more time for clinical supervision. These resources, when mutually recognized and standardized, will also help establish a common baseline prerequisite for training programs across the region, accommodating resident training in different countries as discussed above.
The IAEA has developed a distance learning course to supplement the education and training in programs with limited access to expertise. The Applied Sciences of Oncology distance learning course currently covers 80 modules and has been updated several times since its first release in downloadable CD-ROM format in 2004.29 The modules covered include a wide range of topics from functional anatomy to burnout and coping with patient death and dying. The modules have been downloaded more than 1100 times in the first year after they were launched and are now available as courses in the IAEA’s open e-learning platform.30
When more such resources are available, officially recognized, and continuously maintained, they will be valuable resources to support curriculum harmonization. Such resources will provide a common basic standard for the prior learning done by a foreign candidate. This allows training programs to use such distance learning resources as prerequisites before accepting foreign trainees. This could potentially shorten training time away from the country of origin and reduce overall training cost.
To further improve harmonization and reduce the need for trainees to train abroad, an online learning environment can be developed. This online platform will allow trainees, staffs and programs from different countries in the region to interact, share expertise and collaborate, forming a virtual “regional training program.” One example is the e-learning platform for Advanced Medical Physics Learning Environment (AMPLE), which was designed and piloted under a Regional Technical Cooperation project in Asia Pacific to support training programs in implementing the IAEA syllabus and guidance documents for the education and training of medical physicists. The platform, based on Moodle and hosted on the IAEA e-learning site, provided a centralized electronic record of training and assessment, linked sub-modules with learning resources, and promoted communication and collaboration through online communication tools. A particularly encouraging observation from the pilot project was that AMPLE enabled medical physicists from one country to assist in the supervision of trainees in neighboring countries, allowing a regional sharing of teaching workload and expertise.31 Work is now underway to develop similar online platforms for the education and training of radiation oncologists (Advanced Radiation Oncology Learning Environment, AROLE) and Radiation Therapy Technologists (Advanced Radiation therapy Technology Learning Environment, ARTTLE). Additional tools such as discussion forums, online journal clubs, and shared repositories would further encourage collaboration.
Limitations
The EBEST method we used in this analysis allowed us to estimate the future needs in radiation therapy equipment and radiation oncologists. However, this method has the potential to overestimate the actual demands, risking excess capacity.12 Unfortunately, it is currently the best method we have to estimate the need in lower-middle- and low-income countries where data is limited and access to radiation therapy is inadequate. The “lower estimate” based on 26% RTUs that we used in this analysis is included to provide a safety margin to avoid gross overestimation. The number is also very close to the median actual radiation therapy utilization rate (aRTU) of 28% in a recently published survey of nine middle-income countries.32 Most of the strategies we describe in this article assume that although the optimal number is beyond reach, this “lower estimate” is reasonably achievable.
Conclusions
While the cost and complexity of radiation therapy machine infrastructure has been well-documented, our analysis shows that radiation oncologist training will be equally important to ensuring access to radiation therapy. A significant deficit in trained radiation oncologists in lower-middle- and low-income countries will likely persist and widen in 2030 unless alternative strategies are pursued. Upper-middle and high-income countries may have a substantial role in training the global radiation oncology workforce. Systematic, scalable, and mutually supported training and accreditation strategies are needed. Collaborative, e-learning platforms in combination with traditional, apprenticeship-based, in-person training are required to maximize learning efficiency and minimize costs. Additionally, strategies to optimize migration and incentivize trainees to practice in lower-middle- and low-income countries are needed. Finally, while this analysis focused on radiation oncologists, multidisciplinary training for medical physicists, radiation therapy technologists, and radiation oncology nurses will be essential to realize global access to radiation therapy.
References
- Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiation therapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045 (15)00222-3.
- The Global Cancer Observatory (GCO). http://gco.iarc.fr/. Accessed June 15, 2019.
- Abdel-Wahab M, Bourque J-M, Pynda Y, et al. Status of radiation therapy resources in Africa: an International Atomic Energy Agency analysis. Lancet Oncol. 2013;14(4):e168-e175. doi:10.1016/S1470-2045(12)70532-6.
- Zubizarreta EH, Fidarova E, Healy B, Rosenblatt E. Need for radiation therapy in low and middle income countries - the silent crisis continues. Clin Oncol (R Coll Radiol). 2015;27(2):107-114. doi:10.1016/j.clon.2014.10.006.
- Elmore SNC, Grover S, Bourque J-M, et al. Global palliative radiation therapy: a framework to improve access in resource-constrained settings. Ann Palliat Med. 2019;8(0):202-202. doi:10.21037/apm.2019.02.02.
- Rosenblatt E. Planning national radiation therapy services. Front Oncol. 2014;4(Suppl 6):315. doi:10.3389/fonc.2014.00315.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492.
- World Bank national accounts data and OECD National Accounts data files. https://data.worldbank.org/indicator/ny.gnp.atls.cd. Accessed June 15, 2019.
- Delaney G, Jacob S, Featherstone C, Barton M. The role of radiation therapy in cancer treatment. Cancer. 2005;104(6):1129-1137. doi:10.1002/cncr.21324.
- Wong K, Delaney GP, Barton MB. Evidence-based optimal number of radiation therapy fractions for cancer: A useful tool to estimate radiation therapy demand. Radiother Oncol. 2016;119(1):145-149. doi:10.1016/j.radonc.2015.12.001.
- Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiation therapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024.
- Mackillop WJ, Kong W, Brundage M, et al. A comparison of evidence-based estimates and empirical benchmarks of the appropriate rate of use of radiation therapy in Ontario. Int J Radiat Oncol Biol Phys. 2015;91(5):1099-1107. doi:10.1016/j.ijrobp.2014.11.026.
- Barton MB, Gabriel GS, Delaney GP. Testing criterion-based benchmarking for the appropriate use of radiation therapy. Radiother Oncol. 2018;128(3):406-410. doi:10.1016/j.radonc.2018.05.023.
- DIRAC (DIrectory of Radiotherapy Centres). http://www-naweb.iaea.org/nahu/dirac/default.asp. Accessed June 15, 2019.
- Bibault J-E, Franco P, Borst GR, et al. Learning radiation oncology in Europe: results of the ESTRO multidisciplinary survey. Clinical and Translational Radiation Oncology. 2018;9:61-67. doi:10.1016/j.ctro.2018.02.001.
- Eriksen JG, Beavis AW, Coffey MA, et al. The updated ESTRO core curricula 2011 for clinicians, medical physicists and RTTs in radiation therapy/radiation oncology. Radiother Oncol. 2012;103(1):103-108. doi:10.1016/j.radonc.2012.02.007.
- ACGME Program Requirements for Graduate Medical Education in Radiation Oncology. 2019 ed. Accreditation Council for Graduate Medical Education https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/430_RadiationOncology_2019_TCC.pdf?ver=2019-03-21-164425-110. Accessed June 15, 2019.
- European Society for Therapeutic Radiology and Oncology. Recommended ESTRO Core Curriculum for Radiation Oncologists/Radiotherapists. 3rd ed.; 2010:1-34.
- The Royal College of Radiologists. Specialty Training Curriculum for Clinical Oncology. December 2016:1-36.
- International Atomic Energy Agency. IAEA Syllabus for the Education and Training of Radiation Oncologists. August 2009:1-46.
- Pan HY, Haffty BG, Falit BP, et al. Supply and demand for radiation oncology in the United States: updated projections for 2015 to 2025. Int J Radiat Oncol Biol Phys. 2016;96(3):493-500. doi:10.1016/j.ijrobp.2016.02.064.
- The Royal College of Radiologists. Clinical Oncology UK Workforce Census Report 2018. 2019:1-45.
- Loewen SK, Doll CM, Halperin R, et al. Taking Stock: The Canadian Association of Radiation Oncology 2017 Radiation Oncologist Workforce Study. Int J Radiat Oncol Biol Phys. May 2019. doi:10.1016/j.ijrobp.2019.04.035.
- Accreditation Council for Graduate Medical Education (ACGME). Advancing Innovation in Residency Education (AIRE). https://www.acgme.org/What-We-Do/Accreditation/Advancing-Innovation-in-Residency-Education-AIRE. Accessed June 15, 2019.
- Kerr SP, Kerr W, Ozden C, Parsons C. Global Talent Flows. October 2016:1-36.
- Mathews M, Heath SL, Neufeld SM, Samarasena A. Evaluation of physician return-for-service agreements in Newfoundland and Labrador. Healthc Policy. 2013;8(3):42-56.
- Stilwell B, Diallo K, Zurn P, Vujicic M, Adams O, Dal Poz M. Migration of health-care workers from developing countries: strategic approaches to its management. Bull World Health Organ. 2004;82(8):595-600.
- Fedyanova Y. Incentivizing young doctors to practise in underserved areas. Canadian Medical Association Journal. February 20, 2018: E203-E203.
- Barton MB, Thode RJ. Distance learning in the Applied Sciences of Oncology. Radiother Oncol. 2010;95(1):129-132. doi:10.1016/j.radonc.2010.02.011.
- International Atomic Energy Agency. The Applied Sciences of Oncology (ASO) Distance-Learning Course. https://elearning.iaea.org/m2/course/index.php?categoryid=45. Accessed June 15, 2019.
- Pawiro SA, Lee J, Haryanto F, et al. Current status of medical physics recognition in SEAFOMP countries. Med Phys Int J. 2017;5(1):11-15.
- Rosenblatt E, Fidarova E, Zubizarreta EH, et al. Radiation therapy utilization in developing countries: an IAEA study. Radiother Oncol. 2018;128(3):400-405. doi:10.1016/j.radonc. 2018.05.014.
Citation
SNC E, GB P, JAP R, E Z. The global radiation oncology workforce in 2030: Estimating physician training needs and proposing solutions to scale up capacit. Appl Radiat Oncol. 2019;(2):10-16.
July 11, 2019