The Use of Adaptive Radiation in a Retroperitoneal Seminoma Patient with Poor Candidacy for Chemotherapy: A Teaching Case
Affiliations
- University of Alabama at Birmingham Heersink School of Medicine, Birmingham AL
- University of Alabama at Birmingham Hospital Department of Radiation Oncology, Birmingham AL
Testicular cancers are a common etiology for painless testicular masses occurring in young adult males between the ages of 20 and 34, with 50% diagnosed as seminomas. Seminomas are a malignant germ-cell tumor most commonly confined to the testicles with less frequent de novo involvement of structures outside of the seminiferous tubules. Radiation therapy (RT) has historically been indicated for patients with stage 1-2 seminoma, but its use is declining due to increased uptake of surveillance and modern chemotherapy. However, seminoma is a radiosensitive tumor, and RT may play a role in select cases. This case report describes a nontypical presentation of a large IIC retroperitoneal seminoma measuring 15 cm in an older patient who was a suboptimal candidate for chemotherapy. He was treated with an online adaptive external-beam radiation platform, leading to a significant reduction in body and bowel dose compared with standard-of-care radiation treatment. Clinical regression of tumor was achieved while sparing exposure to significant volumes of bowel and healthy pelvic tissue. The patient had an uneventful recovery and showed no signs of radiation dermatitis or gastrointestinal toxicity.
Case Presentation
A 71-year-old man with a history of infantile left-sided cryptorchidism that he believed to have been resolved via left-sided orchiectomy at age 15 brought with him a CT scan from a community hospital revealing ipsilateral retroperitoneal mass to presentation at our institutional emergency department for evaluation of back pain and a growth in his groin. Scrotal ultrasound revealed one normal right testicle and an apparent left testicle in the left inguinal canal with at least one irregular, hypoechoic mass measuring 1.0 × 1.3 × 1.1 cm. Color Doppler flow was present within this mass. An additional separate hypoechoic mass inferior to the testicle within the inguinal canal that measured 3.0 × 2.3 × 2.5 cm was also noted. CT of the abdomen and pelvis revealed a large retroperitoneal mass measuring 15.0 × 11.3 × 7.6 cm ( Figure 1 ). Of note, the presence of a left testicle in the inguinal canal was unexpected given the patient’s report of his past medical history, and he was understandably surprised by its identification. An atrophic left kidney with associated hydronephrosis and microlithiasis was also noted. Serum labs, including Complete Metabolic Panel revealed serum creatinine of 1.9 mg/dL (normal: 0.74-1.35 mg/dL), estimated Glomerular Filtration Rate (eGFR) of 37 mL/min/1.73 m² (normal: > 90 mL/min/1.73 m²), consistent with significant renal impairment, as well as alpha-fetoprotein of 1.87 ng/mL (normal: < 10 ng/mL), lactate dehydrogenase of 189 IU/L (normal: 135-225 U/L), and beta-hCG of 4.3 mIU/mL (normal: < 2 mIU/mL), consistent with the criteria for pure seminoma.
CT of the abdomen and pelvis revealing the patient’s large retroperitoneal mass
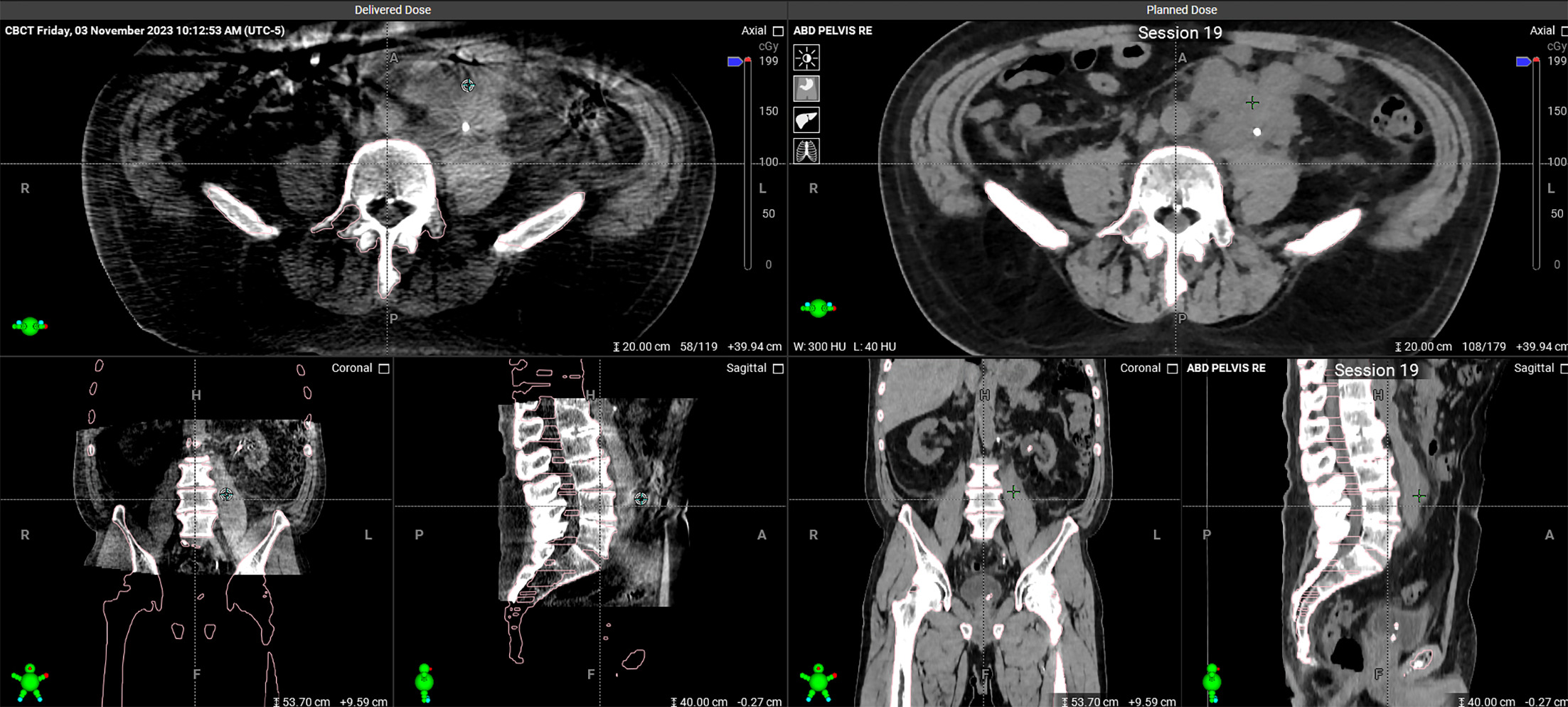
Subsequently, the patient underwent left-sided ureteral stent placement and left-sided radical orchiectomy with high ligation of the spermatic cord. Pathology of the testicle and paratesticular mass revealed seminoma measuring 2.7 cm with focal necrosis and confined to the testes. While pathology did identify 4.0 cm of discontinuous spermatic cord involvement, which typically corresponds with stage 3 malignancy, there was no lymphovascular invasion appreciated and margins were negative. The tumor was therefore staged as pT3N0M1, with an overall staging consistent with a bulky stage 2 seminoma. The patient was evaluated by our institutional medical oncology service, and he was determined to be a suboptimal candidate for cisplatin-based therapy due to advanced age and renal impairment as evidenced by his eGFR. As such, he was referred to the Department of Radiation Oncology, where he was counseled regarding the details of treatment, including adverse effects, risks, and benefits, and gave his consent. After planning, his case was reviewed at our multidisciplinary genitourinary tumor board meeting.
The kilovoltage cone beam CT (kV-CBCT)-guided online adaptive radiation therapy (OART) workflow using Ethos starts by generating a reference plan on the simulation CT image and PET fusion imaging in the supine position prior to an initial treatment course of adaptive external beam radiation (photon- 6X-FFF). Simulation and treatment were in accordance with the previously described institutional workflow by Stanley et al, which includes a comprehensive protocol for integrating kV-CBCT into OART. The implementation process begins with the setup of kV-CBCT systems and the development of standard operating procedures. The workflow involves initial imaging with kV-CBCT to assess anatomical changes at sequential visits and ensure accurate tumor targeting. The kV-CBCT scan was acquired and influencers, that is, structures critical for guiding image deformation, were automatically contoured (bowel, liver, kidneys, etc.). The influencers were further modified based on real-time data by the qualified medical physicist (QMP) as necessary to account for any deviations from the planned treatment field and reviewed by the treating physician once weekly. This adaptive approach, coupled with regular quality assurance checks, functions to maintain system accuracy and effectiveness. In this patient’s case, the primary gross tumor volume (GTVp), low-dose clinical treatment volume (CTV_Low), and other normal tissues were then propagated via a deformable image registration of the simulation CT to the daily kV-CBCT, followed by edits by the QMP or treating physician as necessary. The CTV_High, PTV_Low, and PTV_High were then derived from the GTVp and CTV_Low using prescribed derivations. Two plans were then generated: a scheduled plan (reference plan recalculated onto daily anatomy) and an adaptive plan (new plan optimized using daily anatomy). The superior of the two was then selected for treatment. Lastly, a verification kV-CBCT was taken after the adaptive planning process and immediately before treatment for patient alignment. IDENTIFY surface monitoring (Varian) was utilized to monitor patient motion throughout the entire treatment session.1 The process of refining the technology and workflow based on clinical feedback and technical challenges encountered is crucial to the development and success of a quality support system for troubleshooting and optimization during the course of a patient’s individualized treatment plan.1, 2
For the adaptive therapy in this particular case, GTVp and CTV_Low were drawn each day. CTV_Low consisted of the gross tumor, para-aortic lymph nodes, and ipsilateral lymph nodes extending from the top of the femoral heads inferiorly to the renal vessels superiorly. Note that planning did not include exposure to the inguinal lymph nodes as the rates of primary spread in this setting are low, even in patients with previously violated vascular planes, such as in patients with a surgical history of orchiopexy. The risk of leaving inguinal lymph nodes untreated is further lessened when resection measures include high ligation of the spermatic cord, as was the case with this patient.3, 4 The CTV_High was a 10 mm expansion of the GTVp_High, excluding the bowel. The CTV-PTV margin for both high- and low-dose volumes was 8 mm. The PTV_Low was prescribed a dose of 2520 cGy in 14 fractions, followed by a sequential 1980 cGy boost in 11 fractions to the PTV_High.
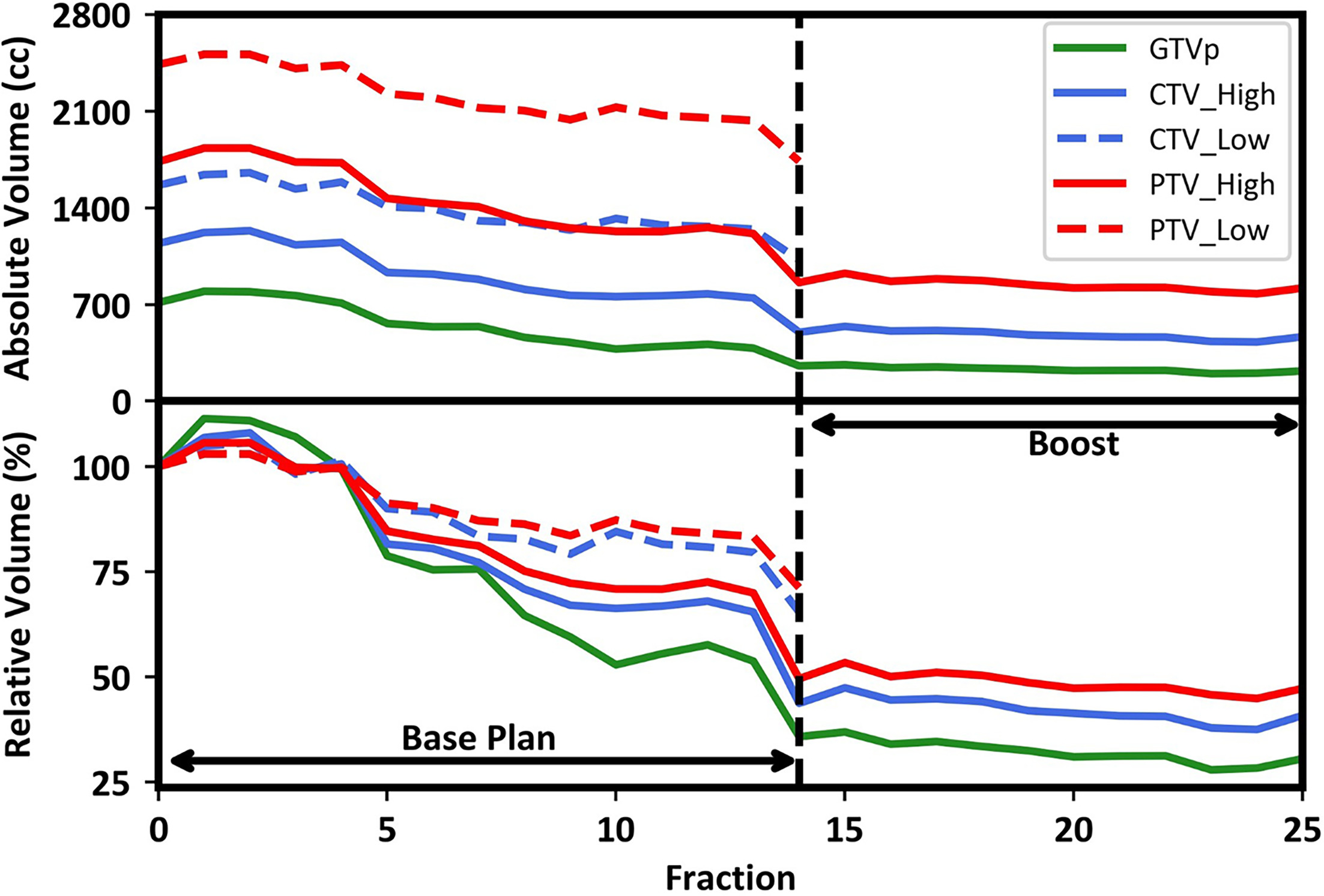
During radiation therapy, a significant reduction in gross tumor volume was appreciated, with reductions in clinical treatment volume from the initial standard plan to the adapted final treatment plan, as shown in Figure 2 . The increase in volume treated at fraction 1 compared with reference volume measured during the initial simulation demonstrates rapid neoplastic growth. Initial GTVp had a volume of 713.88 cm3 with treatment volumes of 795.5 ccs, 263.2 cm3 , and 217.8 cm3 at fractions 1, 15, and 25, respectively, resulting in a 70% total volume reduction by the end of treatment. Similarly, PTV_High had a reference volume of 1734.4 cm3 with treatment volumes of 1833.4 cm3 , 859.4 cm3 , and 819.2 cm3 for fractions 1, 14, and 25, respectively, resulting in a 53% total volume reduction. Also noteworthy is the trend for PTV_High volume reduction at fractions 5, 7, and 14, indicating points of the greatest volume reduction. The trend in GTVp volume reduction at fractions 5, 10, and 14 indicates points of the greatest volume reduction. These trends can be similarly appreciated in all measured volumes in Figure 2 . Figure 3 shows that without adaption the prescription-level isodose would have been overcovered by 66.3 cm3 and 41.6 cm3 at fractions 15 and 25, respectively. Although not explicitly displayed in Figure 3 , the prescription-level isodose would have been overcovered by 3.9 cm3 in fraction 21 without adaption. However, with adaption, the size of the radiation treatment field decreased with the targets, sparing the areas where the tumor regressed. Note that the patient was treated with the adaptive plan for 22 out of 25 fractions. The nonadaptive fractions were delivered on fractions 2, 3, and 5.
Absolute and relative target change, with fraction zero showing volumes at simulation
Isodose color wash from reference plans and subsequent fractions as seen on the planning CT and adaptive CBCTs, respectively. The GTVp is shown in green, CTV_High and CTV_Low in blue, PTV_High and PTV_Low in red, and bowel in purple. Starting from fraction 15, which was the first fraction for phase 2, only GTVp, CTV_High, and PTV_High are shown. Relative values for isodose color wash correspond to 49.5, 45, 42.75, 40, 30, 25.2, and 23.9 Gy when shown on the plan sum.
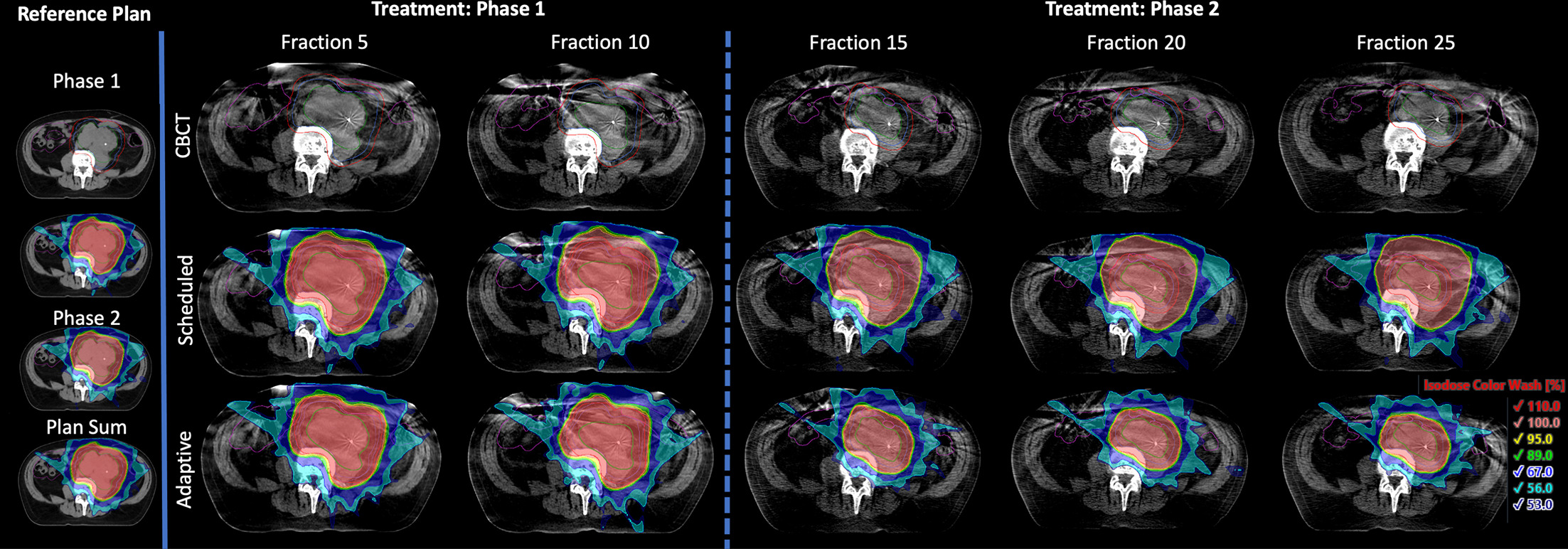
The patient tolerated treatment well and had an uncomplicated recovery. He was evaluated regularly in an outpatient clinic during the 5-week course of his treatment, and apart from preexisting restriction in physically strenuous activity due to advanced age, he tolerated the treatment well. He showed no signs of radiation dermatitis or gastrointestinal toxicity and thus required no additional pharmacotherapies. After the completion of radiation therapy, MRI and CT scans confirmed clinical regression of disease from 795.5 cm3 at induction of therapy to 217.8 cm3 .
Discussion and Conclusions
The optimal management for patients with seminoma is continuing to evolve with increasingly tailored treatment with the dual goals of maintaining excellent oncological outcomes while mitigating potential treatment-related effects of therapy such as secondary malignancy. Considering recent clinical data including SAKK 01/10, there has been renewed interest regarding the addition of focal radiation with reduced intensity chemotherapy for stage 2A/B patients in concordance with the above directives.5 Adaptive radiation has the potential to further improve the therapeutic ratio for these patients as well as those who are not able to receive systemic therapy by reducing radiation doses to organs at risk including the bowel.3, 6
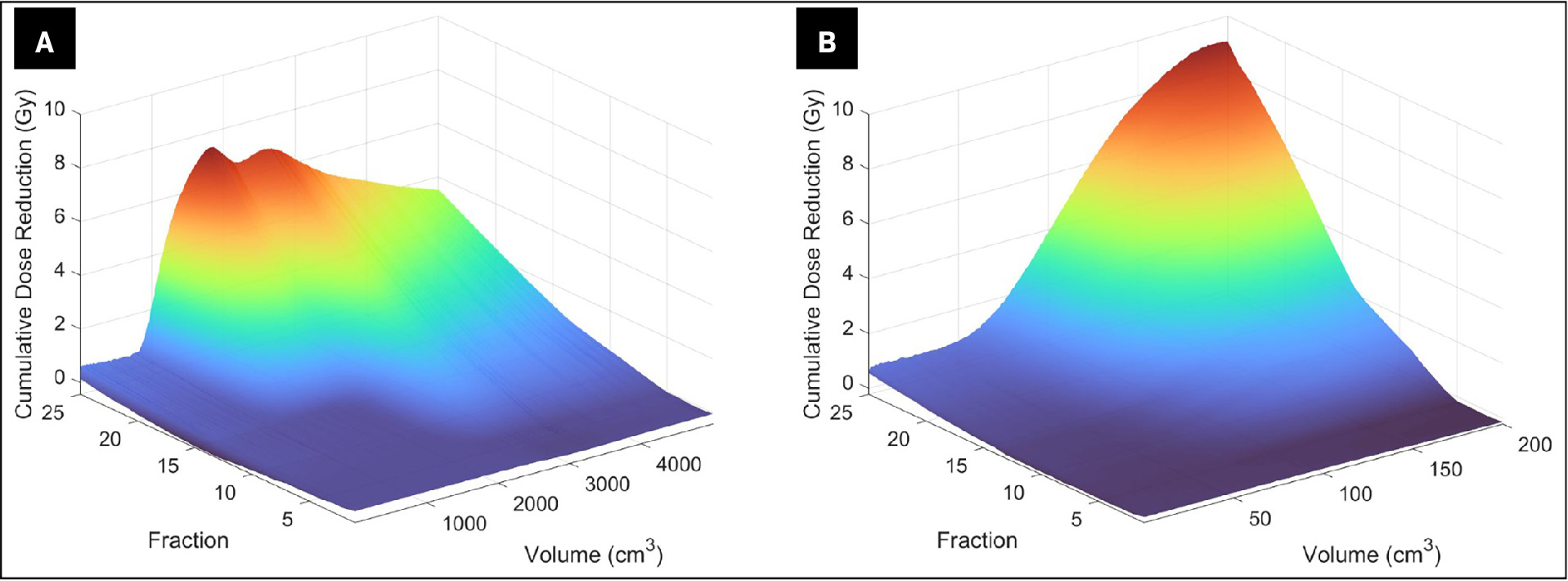
While seminomas are known to be radiosensitive neoplasms, surgical resection and chemotherapy are both preferred to radiation in most young patients due to the risk of late effects, including secondary malignancy. Given the patient’s renal impairment and age, radiation therapy was recommended as the favored curative intent modality7 . Additional considerations for treatment planning involved implementing a dose of 45 Gy rather than the lower doses of 30 or 36 Gy typically employed for bulky stage 2B disease and stage 2C disease, with tumors measuring less than 10 cm.8 The rationale for escalating beyond 36 Gy was established prior to the development of modern chemotherapy and lies in the benefit for patients with a larger treatment volume who are ineligible for chemotherapy and old enough that an exposure of 45 Gy to organs at risk would pose a low risk of late adverse effects such as secondary malignancy. This dose escalation is similar in practice to lymphoma dosing where escalation beyond 30-36 Gy to 40-50 Gy can be reasonably considered in select patients.9, 10 However, the most impactful consideration was the prediction that this large mass in close proximity to a significant portion of the bowel would shrink rapidly during treatment, leaving nearby bowel at risk for toxicity from exposure if an adaptive plan were not implemented. Classically, retroperitoneal radiation therapy is associated with severe nausea, and the degree of nausea is thought to have a directly proportional relationship with the amount of bowel exposure. Therefore, the benefit of utilizing adaptive radiation planning in this patient proved to be the dramatic reduction in bowel and body exposure, which might be responsible for the patient’s reported absence of nausea. Figure 4 shows the cumulative dose sparing as a function of volume and fraction number during adaptive therapy. This sparing is calculated as the difference between the scheduled plan, which represents the therapy that the patient would have had without adaption, and the adapted plan, which was administered to the patient. Note that the tumor enlargement between simulation and the first fraction was anticipated, and fraction 1 was adapted to account for this growth via measurements from CT imaging taken immediately prior. Figure 4 shows that after about the first week of treatment at fraction 5, sparing was noticed across several volumes of bowel and body. After fraction 25, the dose given to the most exposed 50 cc of the bowel — likely the closest segment of bowel to the PTV — was reduced from approximately 4500 cGy to 3500 cGy. If expanded to the most exposed 200 cc closest to the PTV received, a 9000 cGy exposure to organs at risk could be avoided. Reasonably asserting that this number approximates the 45 Gy prescription dose in the scheduled plan, it follows that this segment of the bowel received approximately 36 Gy with the adaptive plan ( Figure 4 ). The use of adaptive radiation in lieu of the standard approach made possible a significantly lower dose delivery to the body, specifically the bowel.
Surface plot of volume receiving cumulative dose reduction equal to cumulative difference between scheduled and adapted — ie, reference plan recalculated on daily anatomy — as a function of fractions for total body ( A ) and bowel ( B )
Tumor regression kinetics for neoplasms traditionally treated with radiation therapy have not been elucidated, so an additional benefit of online adaptive planning is that treatments are continually refined to a shrinking tumor volume. Therefore, when a patient has subpar candidacy for standard treatment methods, adaptive radiation therapy may be a worthwhile option for patients with radiosensitive neoplasms where organs at risk are predicted to accumulate a higher exposure as tumors regress. Further exploration is needed to elucidate trends in the kinetics of tumor regression in radiosensitive neoplasms, which could prove beneficial in an effort for stewardship of the Ethos machine and judicious patient scheduling. Additionally, establishing trends in tumor regression kinetics may justify the addition of an adaptive modification for offline modalities when an online manner is not feasible.
References
Citation
Michayla B. Brown, MS, Mehran B. Yusuf, MD, Joseph M. Harms, PhD, Joel A. Pogue, PhD, Dennis N. Stanley, PhD, Andrew McDonald, MD, MS. The Use of Adaptive Radiation in a Retroperitoneal Seminoma Patient with Poor Candidacy for Chemotherapy: A Teaching Case. Appl Radiat Oncol. 2024;(4):1 - 6.
doi:10.37549/ARO-D-24-00016
December 1, 2024