VMAT: The next generation of IMRT
Images
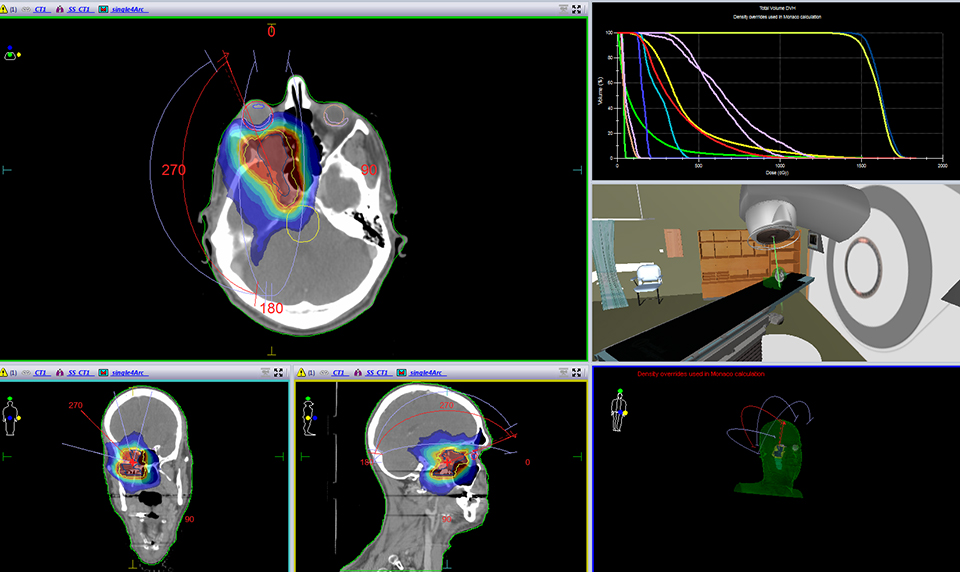
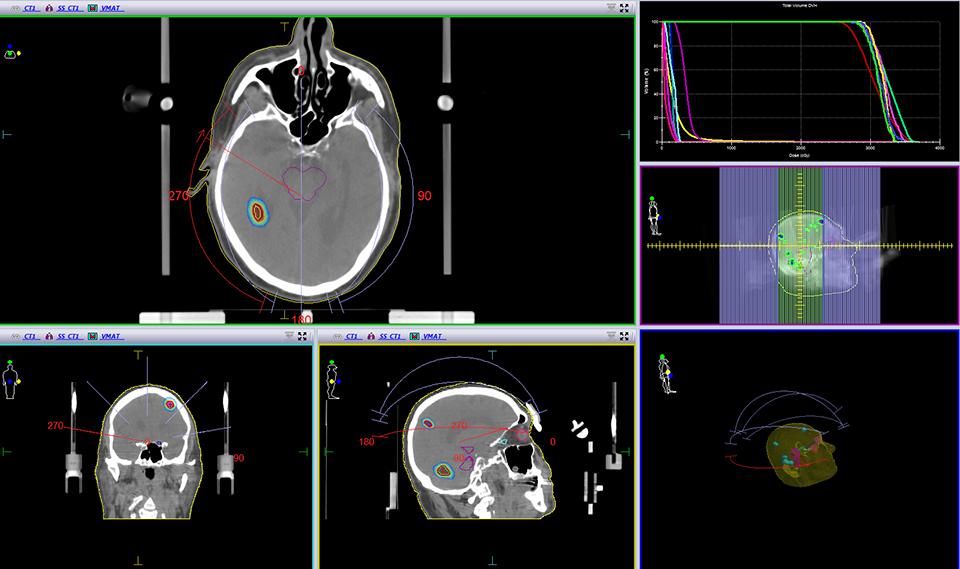
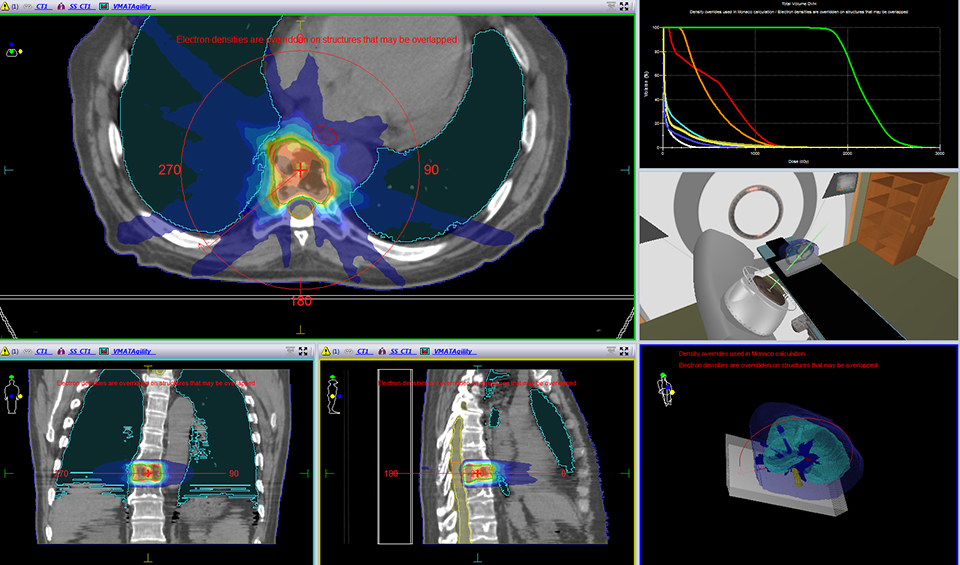
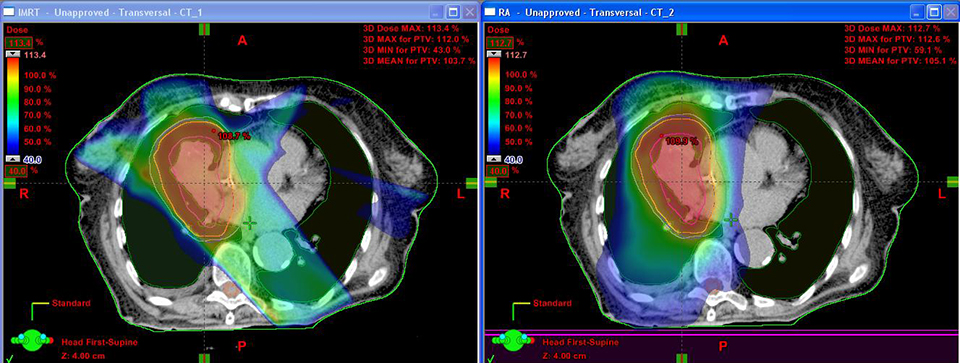
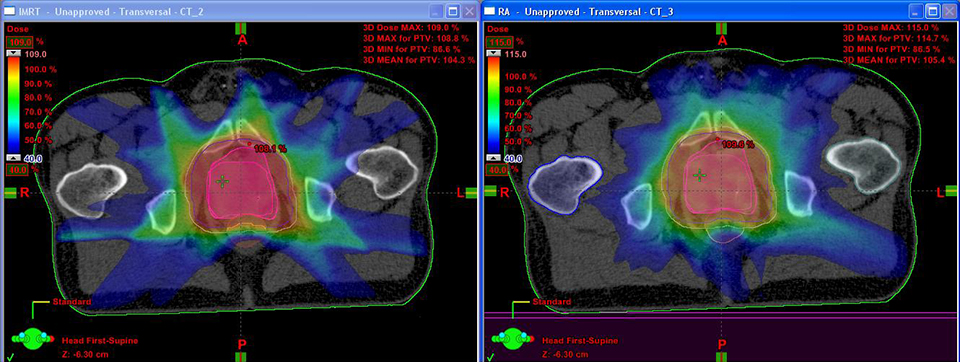
For the past two decades, cancer death rates have steadily dropped, resulting in a 20% plunge in the risk of dying from cancer, according to the American Cancer Society.1 Greater access to cancer prevention, early detection and treatment have played a key role in this decline. However, incidence rates over the last 5 years have essentially remained flat—falling just 0.6% for men and remaining stable in women—calling for the continued development of new and novel treatment strategies.
One such advance is volumetric-modulated arc therapy (VMAT). Originally proposed as intensity-modulated arc therapy (IMAT) in 1995 as an alternative to tomotherapy, the idea of VMAT is to optimize the treatment plan in many angles, and then sequence it into stacks of apertures at every angle, followed by delivery of the beam with multiple connected arcs.2 VMAT delivers radiation with a multileaf collimator in a continuous dynamic mode during a single (or multiple) rotation of the gantry.
The early days
Varian Medical Systems (Palo Alto, California) commercialized VMAT in 2008 with the introduction of RapidArc, a single arc solution, followed by Elekta (Atlanta, Georgia), which developed a single and multiple arc solution. Philips Healthcare (Andover, Massachusetts) provides a treatment planning solution, SmartArc, while Siemens Healthcare (Malvern, Pennsylvania) offers a single and multiple arc planning solution, called Prowess. Sun Nuclear Corp. (Melbourne, Florida) also provides QA and dosimetry tools for VMAT treatment plans.
Initially, a key hindrance to VMAT’s clinical adoption was optimizing the treatment plan, explains Kevin Brown, global vice president of Scientific Research at Elekta. “In the early days of VMAT, the dose distributions were not as good as IMRT,” he explains. “Now that the optimizers have improved, there is no fundamental reason why the dose distributions should not be as good as IMRT.”
In fact, one thing Brown and his researchers learned through their clinical collaborators was the importance of varying dose rate as treatment progressed. That’s when Elekta began referring to IMAT as VMAT, says Brown. Most of the early clinical work on VMAT was for large concave targets, since these cannot be adequately treated with static beams. But for most targets today, clinicians can develop plans with an equivalent dose distribution with IMRT and VMAT.
“The difference is that VMAT will deliver the treatment faster,” says Brown. “Today, it’s a question of why not, rather than why.”
Accelerated treatment, enhanced focus
At the Swedish Cancer Institute in Seattle, Washington, Vivek K. Mehta, MD, a radiation oncologist and director for the Center for Advanced Targeted Radiotherapies, says that in addition to faster treatments, VMAT offers better treatment plans. “With more angles, we can be more focused on the tumor and less on the surrounding healthy tissue.”
In his center, the first in North America to deliver VMAT plans with an Elekta linac, an initial comparison of IMRT to VMAT plans in 100 patients found that 95% were superior with VMAT across all disease types. “As we gained more experience, we re-planned those 100 patients and looked at the 5 where IMRT was better,” says Dr. Mehta.
The result: Today 99% of VMAT plans are superior to IMRT at Swedish Cancer Institute. “There [are fewer] monitor units, better conformality, and it takes less time,” he says.
“VMAT is the next generation of IMRT,” adds Abhi Chakrabarti, PhD, director of Global Marketing for Philips Radiation Oncology Systems. “With VMAT, the technology allows treatments to be given in a shorter time; and therefore, the likelihood of patient movement decreases. The more clinicians can control something that is potentially damaging to healthy tissue—radiation—the more they can use it for the good.”
Dr. Chakrabarti also has seen several centers outside the United States make the leap to VMAT from 3D conformal therapy, without implementing IMRT. “IMRT is more complex with more quality assurance (QA), but does not provide the time benefit,” he says. With VMAT, the quality and time benefits exist, particularly for centers that have a large population base, whether in mature markets or emerging markets.
At Lewis Gale Regional Cancer Center in Pulaski, Virginia, James Nunn, MS, CHP, DABR, senior medical physicist, has seen firsthand the efficiency and speed of treatment with VMAT. Treatment times for patients receiving 7 to 14 individual beams with IMRT, especially if utilizing split beams, can take 30 minutes, he explains. VMAT can help lower a delivery from several minutes with IMRT down to 1 to 2 minutes per arc. That efficiency, and the potential to treat more patients without extending the clinic’s hours, makes VMAT an economically attractive solution for busy facilities.
The question of integral dose
In addition to shrinking treatment times, another VMAT advantage is that it allows the clinician to shape the dose more conformally to the target’s location, says Deepak Khuntia, MD, a radiation oncologist at the Targeted Radiation Institute, Pleasanton, California, and vice president of Medical Affairs with Varian Medical Systems. “While we can get more conformal plans than before, the integral dose—the total dose of radiation absorbed by the body—is more spread out than it would be with conventional IMRT and 3D techniques,” he says, “and we must pay close attention to that.”
While there is limited evidence that higher integral dose impacts patient outcomes, clinicians should review this consideration on a case-by-case basis to ensure doses to normal structures are low enough to meet practice standards.
Nunn agrees that integral dose is an important consideration when developing treatment plans with modulated arcs. With a traditional IMRT treatment using 5 to 7 beams, some areas in the body receive little radiation. As such, the integral dose is very low. With VMAT, however, the arc is continually moving as the multileaf collimator (MLC) modulates dose. As a result, some areas receive a radiation dose that they otherwise would not with traditional IMRT techniques.
“With VMAT, we have dose going through the body at 360°, so integral dose becomes a more important factor in areas with critical structures,” explains Nunn. “This is why in our facility we haven’t switched everything over to VMAT. In some instances the integral dose to critical structures can be higher with VMAT than IMRT; consequently, in those cases we use traditional IMRT.”
However, Dr. Mehta cautions that the issue of integral dose depends on how you look at low dose. In some cases, dose can be less with VMAT compared to IMRT. For example, since VMAT is delivering dose at every angle, each angle is delivering less dose than if the dose were delivered only across 4 angles. Determining which option is better for patients depends on the particulars. If a patient is being re-treated, then the ability to disburse the dose across more angles may be better than using fewer, fixed angles. On the flip side, if a certain path should be avoided due to a critical structure, then a fixed field makes more sense.
Dr. Mehta also notes that with IMRT, a small amount of low-dose radiation leakage occurs when the machine is ramped up in dose and then brought back down to zero. “With VMAT, we turn the machine on one time, so there is less [leakage] of the radiation,” he explains. “For young patients, we really don’t want any of that low dose leakage.”
VMAT at work
At Lewis Gale Regional Cancer Center, approximately 40 to 60 patients a day receive external-beam radiation therapy (EBRT). Before implementing VMAT, most patients at the clinic received 3D conformal and step-and-shoot IMRT, notes Nunn. Currently, however, VMAT is most often used for treating cancers centrally located in the body, such as the esophagus, prostate, lung and brain.
Nunn adds that with traditional IMRT QA, the accelerator gantry can be held stationary and the plan delivered to a phantom, or chamber array. “You have to be more careful in correcting for how the beam enters your QA device,” he says. “Your QA device placement is, therefore, more critical.”
While the QA process may be more complex, Nunn says VMAT is easier today than when he first used it in 2009. From solutions that check rotational plans, to second-check software with 3D analysis, to new planning software and more advanced computers, Nunn has witnessed several improvements in speed and capability.
“It doesn’t take us too much time to plan arc treatments with today’s computing power, so for some cases we do two plans—IMRT and VMAT,” Nunn explains. “We can then compare target coverage and integral dose, and our physicians can choose the most appropriate plan to meet their treatment objectives…Our learning method was to take our existing IMRT treatment planning knowledge, and extend and modify these techniques to arc treatments.”
A bigger challenge for Nunn was interconnecting devices from various vendors to perform VMAT. While single-vendor solutions are currently available, that wasn’t the case when his facility began acquiring modules to perform VMAT. Nunn had to ensure his second-check software was compatible with arcs, his record-and-verify software could sequence to the linac, and the couch top was properly characterized in the planning software.
For Elekta users, the company’s digital linac helps streamline the move to VMAT. “If our customers have a modern Elekta linac purchased within the last 8 to 10 years, then it is capable of being upgraded to deliver VMAT treatments,” Brown explains. This upgrade is limited to the dose-rate control, and the treatment planning software—the main component. Monaco 5, Elekta’s latest release, features both VMAT and IMRT algorithms.
Elekta’s next generation linac, Versa HD, is further optimized for VMAT treatments. It incorporates the Agility multileaf collimator and the new high-dose-rate mode. According to the company, Agility provides integrated digital control of leaves and leaf guides, combined with unique Rubicon optical leaf positioning for an accurate and reliable beam-shaping solution. Coupled with leaf transmission of less than 0.5 percent, Agility enhances treatment delivery while reducing integral dose.
In 2013, Varian received clearance for RapidPlan, a knowledge-based treatment planning system tool that helps clinics leverage shared clinical best practices from leading institutions, or a center’s own best practices to create a model treatment plan. RapidPlan uses dose and patient anatomy information from existing plans to help clinicians estimate dose distributions in new patients. With RapidPlan, facilities can further decrease variance in the quality of plans, and increase efficiency in the planning process, particularly for complex cases, according to the company. This is not a template, but rather a personalized treatment plan utilizing knowledge obtained from what physicians deem the best plans of the past.
At the American Association of Physicists in Medicine (AAPM) annual meeting in July, Philips introduced Pinnacle3 Auto-Planning, which accelerates both IMRT and VMAT planning and makes the process more consistent and reproducible. The solution reduces time and effort to create a plan, and eliminates manual data entry.
“Clinicians are not only burdened with more patients as volumes increase, but they also want consistency in treatments,” says Dr. Chakrabarti. “We expect variations in skill sets across different centers, and products like this are designed to help elevate the level of the plan for all centers—improving the access and quality of health care for everyone.”
VMAT today and tomorrow
Dr. Mehta and his colleagues have begun using VMAT for stereotactic body radiation therapy (SBRT), and he finds the VMAT plans are comparable to traditional SBRT plans. However, SBRT treatments can take several hours to complete, while VMAT can take several minutes, as in the case of external-beam therapies. This can have a significant impact on lung cancer patients, who often lack good lung capacity and have difficulty holding their breath.
Lung cancer patients can also benefit from triggered imaging, a process that can be used during a VMAT treatment using Varian technology to enhance targeting accuracy during treatment delivery in most disease sites, including the lung. With gold markers implanted into the lung tumor, the patient is imaged at specific points of the respiratory cycle during the VMAT treatment. If the patient moves, the operator can pause the beam and arc until the patient is back in position. The imaging is done in near real time, which enables clinicians to better ensure that radiation is being delivered to the right place at the right time, says Dr. Khuntia.
“The imaging, treatment plan and motion interfaces are all put together in a harmonic way to allow the operator to analyze each component at once and prevent mistreatment,” he says.
Looking to the future, Nunn expects to see more VMAT treatment techniques used for stereotactic ablative radiotherapy (SABR) treatments. Another prediction is that VMAT will replace most IMRT plans in the United States, says Dr. Mehta.
“Many centers will find the leap to VMAT from IMRT is not that hard…Once they have the skill set for IMRT, they can use that same exact skill set for VMAT,” he says. “It’s an evolution, a continuation and improvement to IMRT.”
As automation increases, Brown also predicts greater VMAT adoption. “As we make the entire delivery process more automatic, that will make the process even more efficient, reproducible and safer,” he says. “Clinicians will be looking for the most efficient way to deliver good quality treatments to every patient. VMAT represents, for the vast majority, the most efficient way to deliver treatment.”
References
- Siegel R, Ma J, Zou Z et al. Cancer statistics, 2014. CA: Cancer J Clin, 2014; DOI:10.3322/caac.21208.
- Yu CX. VMAT and the tradeoff between treatment time and dose conformality. http://vimeo.com/77145182. Paper presented at: American Association of Physicists in Medicine 52nd annual meeting and exhibition, Philadelphia,July 18-20, 2010.