Workflow Considerations for Implementing a Cone-Beam CT-Guided Adaptive Radiation Therapy Program
Affiliations
- 1 Department of Radiation Oncology, Washington University School of Medicine in St. Louis, St. Louis MO
- 2 Department of Radiation Oncology, University Hospitals/Case Western Reserve University, Cleveland OH
- 3 Department of Radiation Oncology, Keck School of Medicine of USC, Norris Comprehensive Cancer Center, Los Angeles CA
Cone-beam CT-guided adaptive radiation therapy (CTgART) is an emerging treatment paradigm that enables the delivery of online adaptive radiation therapy (ART) using CT-based onboard imaging. Our department installed and implemented a CTgART system in 2019 and has since developed workflows for the delivery of CTgART to a wide variety of disease sites. Herein we describe workflow considerations for implementing a CTgART program with a specific focus on the template-based treatment planning methodology that drives online adaptive plan creation on our department’s CTgART platform (Ethos; Varian Medical Systems, Palo Alto, CA). We describe disease-site-specific information for the delivery of CTgART to the thorax, abdomen, prostate, and bladder, and discuss future directions for this technology, which is becoming increasingly accessible and utilized in radiation oncology.
CME credits available here
Introduction
Online adaptive radiation therapy (ART) utilizes advanced imaging systems and treatment planning techniques to develop a new treatment plan based on the patient’s anatomy of the day while the patient is on the treatment table. The development of stereotactic MR-guided adaptive radiation therapy (MRgART) and cone-beam CT-guided adaptive radiation therapy (CTgART) have allowed for dosimetric improvements, which have led to improved local control and decreased toxicity rates across numerous disease sites.1 - 3 While MRgART has been demonstrated to be an effective tool in the treatment of numerous disease sites, this technology’s wide availability has been limited by resource requirements. More recently, a CTgART platform has been developed and may improve the dosimetric and therapeutic index of radiation therapy in a variety of disease sites.4 - 6 Additionally, one study demonstrated significant cost-savings when using CT-guided stereotactic body radiation therapy (SBRT) compared with stereotactic MRgART (SMART).7
Our department installed a CTgART platform in 2019 and has since developed and tested CTgART workflows in a variety of disease sites. The components of the linear accelerator at our institution (Ethos; Varian Medical Systems, Palo Alto, CA) have been described elsewhere. This platform consists of a ring gantry linear accelerator unit and a dedicated treatment planning system (TPS) that utilizes an Acuros XB, dose-to-medium-based calculation algorithm. The system is capable of intensity-modulated radiation therapy (IMRT) sliding window and volumetric-modulated arc therapy (VMAT) beam delivery. The TPS also utilizes an artificial intelligence (AI)-driven intelligent optimization engine (IOE), which allows users to set hierarchal priorities that define the objective function.8 Use of the IOE is required for all cases by the Ethos system. Once optimization begins, the TPS monitors the plan optimization on its own. Due to the inability to manually change goals during optimization, template-based planning is highly useful in generating clinically acceptable treatment plans.
Onboard image guidance is accomplished using a cone-beam CT (CBCT) system. The rapid nature of CBCT acquisition on this unit paired with the dedicated treatment planning software allows for effective delivery of CTgART.9 - 12 The CTgART workflow includes traditional CT simulation, CBCT-based simulation depending on disease site, traditional treatment planning processes, plan adaptation, and treatment delivery. Once the initial treatment plan has been developed and approved, patients present for adaptive treatment. The online adaptive portion of the workflow includes image acquisition, contouring and online adaptive plan optimization, plan selection, quality assurance (QA), and treatment delivery. Our institution has successfully developed disease site-specific CTgART treatment workflows for pancreatic tumors, abdominal metastases, ultra-central lung tumors, and pelvic malignancies, amongst others.13
In the remainder of this article, we describe simulation, treatment planning, and treatment delivery techniques utilizing CTgART as well as future directions of this rapidly advancing technology.
General CTgART Workflow
Traditional CT Simulation
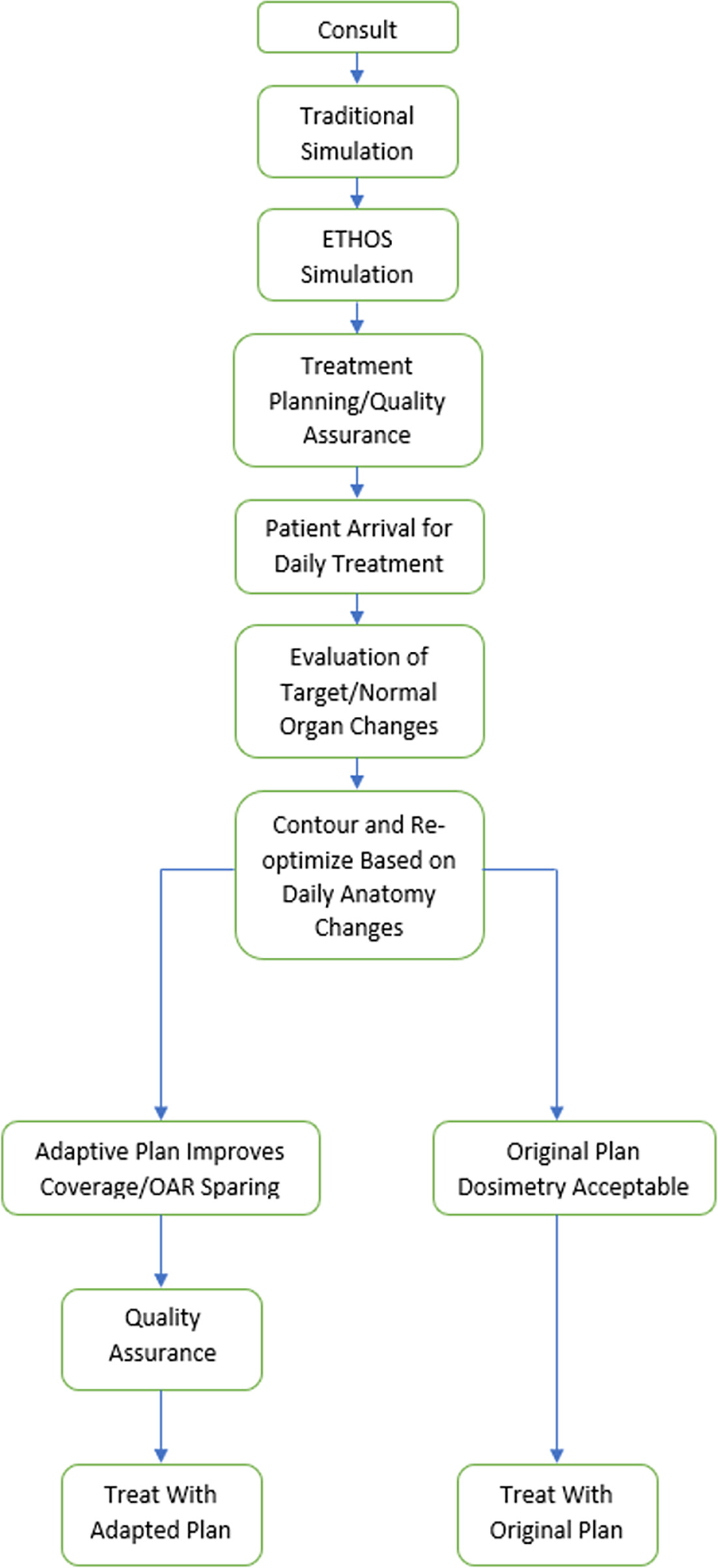
Figure 1 depicts our institution’s CTgART workflow. All patients undergo a traditional CT simulation with various disease site-specific institutional order set protocols with appropriate custom immobilization devices. As ART cases can be fairly time consuming, it is critical that the patient be comfortable in the treatment position for an extended period. For example, for CTgART pancreatic cases, we often simulate with one arm up and one arm down. While traditionally pancreatic radiation therapy is conducted with both arms over head, the one arm up one arm down position is more comfortable for patients to maintain for prolonged online adaptive treatments. Most patients can now be treated with high-quality plans with both arms at their side. Of note, patients are generally scanned (and treated) at breath-hold for thoracic and abdominal CTgART cases.
Adaptive workflow. An overview of the workflow described for treatment with CT-guided adaptive radiation therapy.
CBCT Simulation
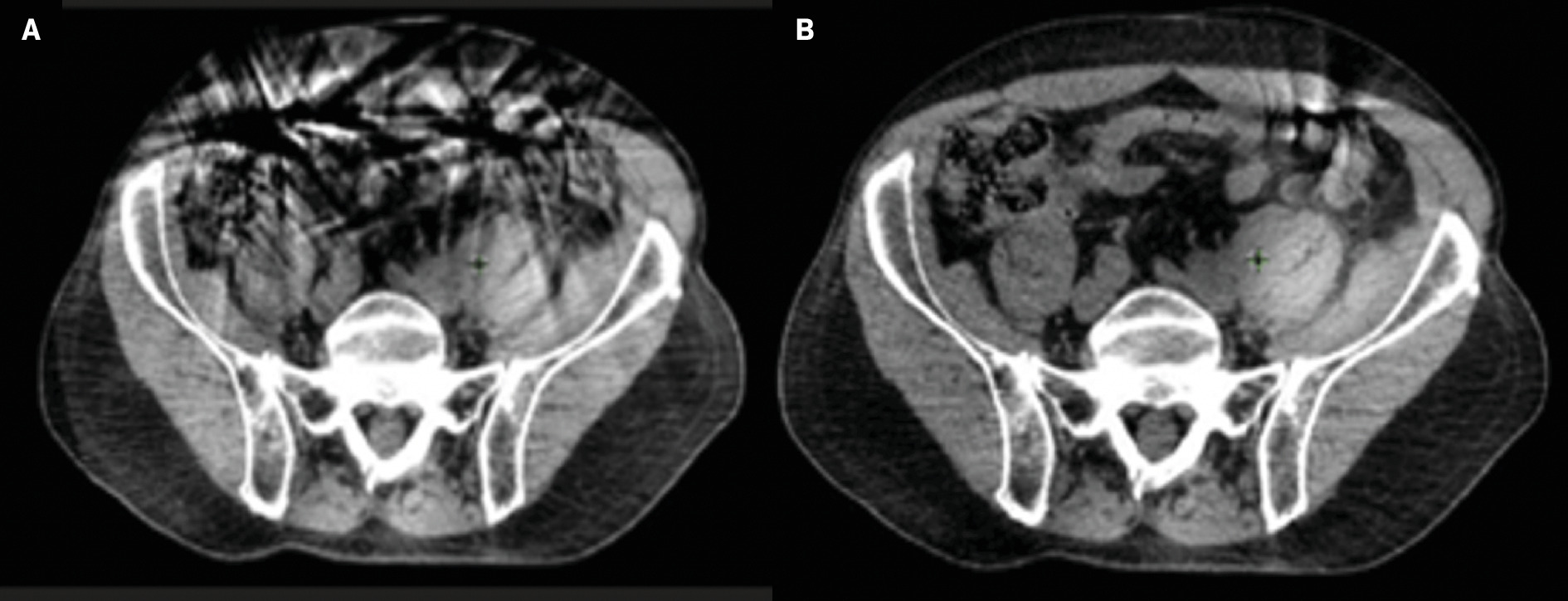
We often subsequently perform a CBCT simulation on our CTgART treatment machine using the same patient positioning and custom immobilization devices. The CBCT simulation is performed to evaluate the patient’s suitability for ART, primarily focusing on the ability to delineate the target and surrounding organs at risk (OARs), and the patient’s ability to perform end-exhale breath-hold in a reliable and reproducible fashion for breath-hold-gated treatments. This is performed for abdominal CTgART SBRT cases, as diaphragmatic motion, abdominal fluid, and breath-hold quality, among other factors, significantly determine CBCT quality and the subsequent ability to perform CTgART. Alternatively, this is not often done for genitourinary and gynecological cases as image quality in the pelvis is not as strongly tied to those factors. Ultimately, the decision to obtain a CBCT simulation in addition to a traditional CT simulation is at the discretion of the treating radiation oncologist and medical physicist as it is specific to each patient’s individual anatomy, regardless of disease site. Figure 2 demonstrates the potential benefits that breath-hold can provide in target and OAR visualization.
Impact of breath-hold on cone-beam CT (CBCT) quality. ( A ) Nonbreath-hold CBCT demonstrating significant artifact. ( B ) Breath-hold CBCT with resultant reduction in artifact.
Treatment Planning
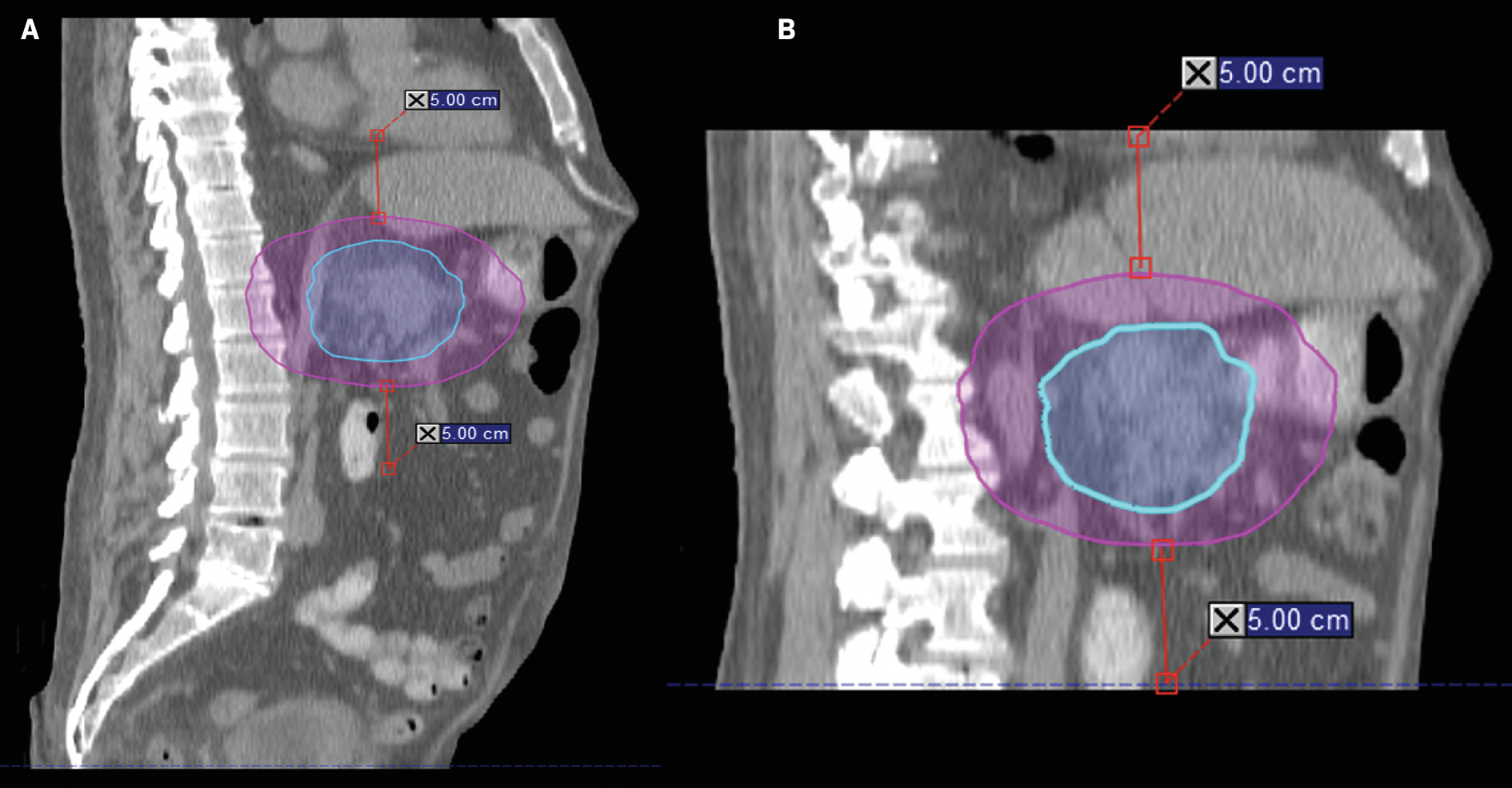
Once simulation imaging has been obtained and contours are complete, a contour ring structure that consists of a site- and patient-specific axial and superior/inferior expansion of the planning target volume (PTV) will be constructed.14, 15 The CT image data set is then shortened to only include image data that spans 5 cm superior and inferior to the contour ring to limit plan optimization time during adaptive treatments, which also assists in minimizing patient time on the table ( Figure 3 ). When examining 10 pancreas SBRT patient plans, we found that the mean VMAT optimization time was 641.53 seconds (~10.7 min) for the full-length CT image data set and only 404.62 seconds (~6.7 min) using the shortened CT. For comparison, IMRT optimization for the same 10 patients took 72.34 seconds and 61.42 seconds for the full data set and shortened data set. Despite the faster optimization time, the mean MU ratio was 8.8 and 4.5 for IMRT and VMAT, respectively. Assuming 800 MU/min dose rate, the mean beam-on time for IMRT and VMAT plans would be approximately 11 minutes and 5.6 minutes, respectively. The choice to use of IMRT or VMAT is site-specific and addressed below.
Example of shortened scan for treatment planning. ( A ) Sagittal view of full-length scan and ( B ) shortened-scan length. Using a shortened scan for treatment planning minimizes optimization time and patient time on the table.
Throughout the history of ART, the goals of adaptation have taken on various forms and today 2 primary strategies exist for ART planning: conventional planning and isotoxic planning.16 In adaptive conventional planning, the goal is to find a balance between coverage and OAR sparing and keep this balance the same throughout treatment.17 In adaptive isotoxic planning, the goal is to dose escalate portions of the PTV that are achievable based on the anatomy of the day while limiting OAR doses to a maximum acceptable constraint. Both account for daily anatomy changes and maintain a level of target coverage, but isotoxic planning target coverage will vary depending on anatomy of the day. For CTgART cases using SBRT dosing (ie, 50 Gy/5 fx), we always use an isotoxic planning strategy to limit normal tissue toxicity.
Template-based planning is the only method for CTgART planning on the Ethos system. During treatment planning, the clinical goals of each structure and their priority are set during the initial template-based planning and cannot be adjusted or edited during an online adaptive session. Therefore, it is imperative that templates be standardized and vetted with a large variety of clinical scenarios to ensure the template (or minor patient-specific changes) can reproducibly provide high-quality, clinically appropriate plans throughout the course of treatment. To verify the quality of a newly constructed template, it is crucial to test the template in silico on a wide variety of previously treated patients.18 - 20 Our process for implementing a new template, which has previously been described, involves rigorous testing of institutionally created templates in prospective in silico imaging studies.9, 13, 19 In these studies, the templates, which are based on adaptive treatment planning procedures and constraints from prior ART and non-ART SBRT treatment planning procedures, are tested within emulated CTgART treatments using images collected from patients being treated with non-CTgART radiation therapy within our department. These in silico studies are typically performed on 7 to 10 emulated patients and are ideally done prior to implementing a new ART workflow of any modality. If the template can construct quality plans among a variety of patients with different anatomies, it will generally be robust when adapting to daily changes in the same patient’s anatomy. To construct robust templates for all disease sites, the prescribing physician must concretely define OAR hard constraints that reflect clinical scenarios in which the oncologist would halt patient treatment if those hard constraints were not met, as well as minimum target coverage goals.
Conventional Approach
When performing CTgART, a conventional treatment planning approach may employ elements such as mean dose constraints to drive plan optimization or have significant OAR and PTV overlap in which a balance of PTV coverage and OAR sparing must be struck. However, the black box nature of the Ethos optimizer limits the usefulness of using generic or conflicting clinical goals (eg, plugging in clinical goals directly from a non-ART trial). In a phantom study, we found that the optimized Dmean dose to OARs overlapping with a PTV does not change monotonically with the Dmean constraints specified in plan directives.8 In simple terms, the IOE can ensure a patient-specific ideal balance of target coverage and OAR sparing for overlapping structures with conflicting constraints using nonoverlapping optimization structures and optimization clinical goals.
Oftentimes conventional planning target prioritizes coverage. However, if the target coverage objective is the topmost priority, OAR sparing will suffer. Commonly in clinical trials, target coverage has 2 levels per protocol and within variation. Variation acceptable allows for cases where a balance can be struck between coverage and sparing. Therefore, in CTgART planning it is helpful to separate the ideal PTV coverage goal and the variation acceptable or minimum target coverage into individual clinical goals. A similar approach can be done with the OARs, with a clinical goal for both an ideal metric and a hard constraint. From here, the clinical goals can be prioritized as follows: (1) minimum PTV coverage, (2) hard OAR constraint, (3) ideal PTV coverage, and (4) ideal OAR constraint. This order allows the optimizer to first prioritize achieving necessary target coverage followed by meeting any hard OAR constraints. To ensure plan robustness, the first 2 goals should never conflict with each other. In cases where they do, the higher priority will trump the lower so that the goal is not met. In general, if the minimum coverage and hard OAR constraint do not conflict, the order should not matter.
Isotoxic Approach
Our clinical implementation of isotoxic treatment planning prioritizes sparing of OARs over PTV coverage through the utilization of treatment planning templates. Our SBRT templates typically include structures such as a PTV_OPT and gross tumor volume (GTV)_OPT in addition to several other ancillary structures, further described in Table 1 . The Opt structures play a key role in plan optimization as they allow for the unique ability to reproduce the optimal plan even in the setting of significant anatomic change. When creating clinical templates for isotoxic treatment planning, it is useful to normalize all plans to meet all priority 1 goals for OARs. By doing so, we can ensure that all OAR hard constraints are met at every fraction when adapting online. To obtain appropriate coverage to the PTV, Opt structures are then set as priority 2 goals. Table 2 depicts sample templated priority 1 and 2 goals that can be utilized when generating adaptive single-dose-level abdominal SBRT plans. Our clinic typically utilizes priority 3 and 4 goals to improve plan quality and optimize structures beyond their hard constraints, if possible. Lastly, “Report Value Only” goals can be set for metrics in which the value is of interest but is not an optimization goal such as PTV V100%Rx.
Optimizations Structures for Template: A Nonexhaustive List of Optimization Structures used in CT-Guided Adaptive Radiation Therapy Treatment Planning, How They Are Created, and Their Purposes
| Structure Name | Structure Creation | Purpose |
|---|---|---|
| ContourRing | An axial and superior/inferior margin from PTV | Defines area where OARs should be recontoured based on daily anatomy |
| PTV_ORT ∑a | PTV cropped out of overlapping OARs + margin | Allows for dose escalation away from critical OARs |
| GTV_OPT ∑ | GTV – OARs + margin | Allows for clinically desirable hot spots within the tumor |
| PTV_OPT_InnerRing ∑ | PTV_OPT – GTV_OPT | Limits hot spots outside of the GTV and promotes dose fall off |
| Control Ring 1 ∑ | 3-5 cm ring around PTV | Helps control low-dose spill and verifies no high dose outside of contour ring for safety purposes |
| NS_TPS_PTV | GTV + radiation oncologist-defined margin | Verifies that derived Ethos PTV structure is consistent with PTV contoured in ARIA (Varian Medical Systems, Palo Alto, CA) and tracks drastic changes in target volume |
a ∑ refers to derived structures that are not hand contoured but rather automatically generated from applying predefined operators on pre-existing additional contours.
Abbreviations : GTV, gross tumor volume; NS, normal structure; OARs, organs at risk; PTV, planning target volume; TPS, treatment planning system.
Abdominal CT-Guided Adaptive Radiation Therapy (CTgART) Template: A Nonexhaustive List of Priority 1 and 2 Goals for the Delivery of Stereotactic Body Radiation Therapy Using a CTgART Platform
| Structure | Goal | Priority |
|---|---|---|
| Stomach | V3300 cGy ≤ 0.50 cm3 | 1 |
| Small bowel | V3300 cGy ≤ 0.50 cm3 | 1 |
| Duodenum | V3300 cGy ≤ 0.50 cm3 | 1 |
| Large bowel | V3300 cGy ≤ 0.50 cm3 | 1 |
| Spinal canal | D0.50 cm3 ≤ 2 500 cGy | 1 |
| PTV_OPT | D95% ≥ 100% | 2 |
| GTV_OPT | V105% ≥ 95% | 2 |
| V120% ≥ 20% | ||
| Both kidneys | Dmean ≤ 1500 cGy | 2 |
| ContourRing | Dmax ≤ 7500 cGy | 2 |
| PTV_OPT_InnerRing | D5% ≤ 110% | 2 |
| PTV_5000 | D95% ≥ 2500 cGy | 2 |
| Liver-GTV | V2500 cGy < 33% | 2 |
PTV, planning target volume.
Plan Optimization and Evaluation
The Dose Preview workspace can be utilized to evaluate potential conflicting objectives that could result in suboptimal plans. This workspace uses a 9 static field fluence-only fast optimization and dose calculation to evaluate the effects of changing clinical goals and their priorities. In CTgART treatments, it can be useful to generate a plan in a standard departmental TPS with a more desirable beam geometry and export the plan to the CTgART TPS for optimization.
CTgART quantitative plan evaluation examines 2 key features of the plan: Does the plan meet all OAR constraints and has sufficient coverage been obtained? The features that influence the quantitative performance of CTgART plans are of the utmost importance and serve as the baseline for assessing plan and template performance. These features include templated OAR constraints and coverage goals defined by the radiation oncologist with knowledge of what is clinically achievable given the clinical patient population. Although these elements should generally remain rigid, user adjustments to these values may be necessary for special patient anatomical consideration.
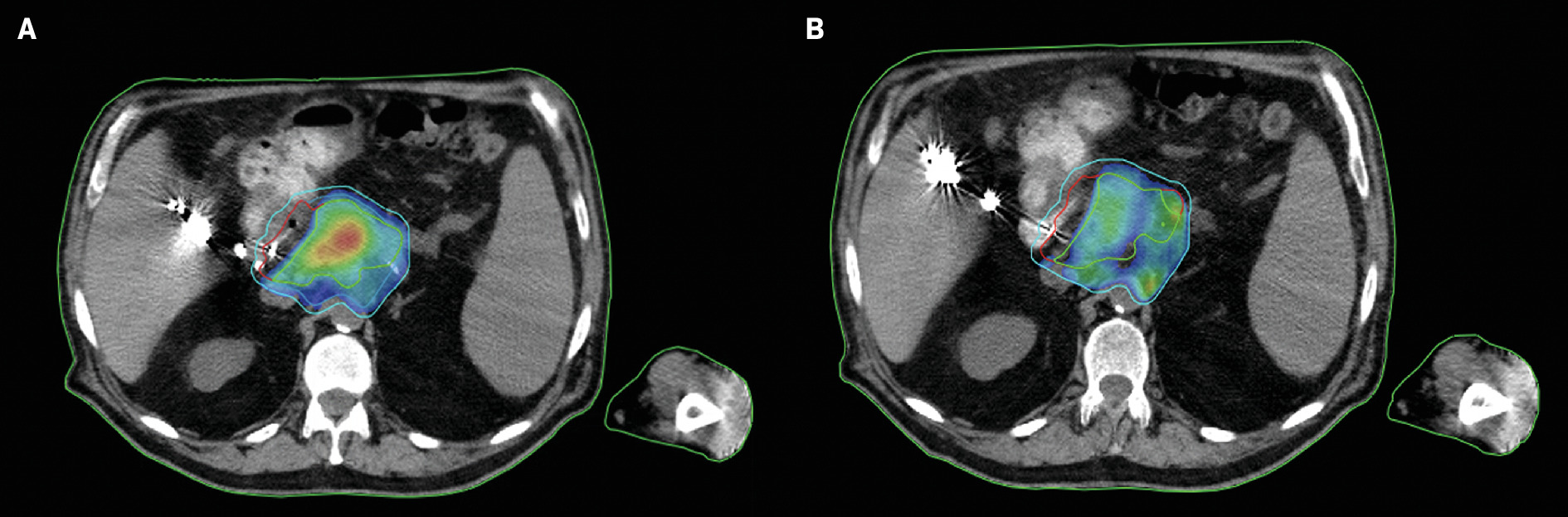
In addition to quantitative performance, it is imperative to assess the plan’s qualitative performance. A robust template should be able to yield a plan that meets clinical constraints; however, meeting clinical constraints alone may not reflect a high-quality plan. The template should include elements that drive the qualitative plan performance where users can adjust the parameters to accommodate patient-specific concerns to obtain improved dose distributions. For example, Figure 4 reflects the impact on plan quality when optimizing with templated goals designed to drive overall plan quality. Notably, both plans meet OAR and PTV_OPT goals; however, additional structures and goals are required to generate SBRT-like dose distributions.
Pancreas CT-guided adaptive radiation therapy plan dose distribution. ( A ) Shown here is the pancreas plan dose distribution when the template includes goals for structures such as GTV_OPT, which are designed to contain the hotspot within the gross tumor volume (GTV) compared with ( B ) the pancreas plan dose distribution without any GTV_OPT goals. The hotspots exist more peripherally, which may be of concern when treating near organs at risk.
Online Adaptive Treatment Delivery Workflow
Our clinical CTgART system is equipped with the HyperSight (Varian Medical Systems, Palo Alto, CA) CBCT technology, which enables the acquisition of CBCT images in 6 seconds with image quality comparable to those acquired from CT simulation.21 Prior to the installation of the HyperSight, all initial CBCTs were taken in breath-hold due to potential motion artifacts to improve image quality, even in pelvic sites which were treated free breathing. With the more advanced imaging hardware, motion artifacts are less of a concern for pelvic treatment sites and initial CBCTs are acquired in the same state as simulation and treatment delivery (free-breathing vs breath-hold).
During daily treatments, CTgART utilizes a serial adaptive workflow in which the default setting is for the initial simulation CT and treatment plan contours to be propagated onto the CBCT anatomy of the day as a means of avoiding propagation of systematic errors that may have been created during previous ART fractions.15 Plan development and reoptimization are performed on the CBCT images.16 If permanent changes need to be saved for further fractions, such as tumor growth, an offline replanning process is required.
When constructing CTgART plans, it is also important to consider isocenter placement to avoid out-of-field artifacts that may arise on the CBCT. The CTgART system will attempt to deform the target volume based on the influencers and deformable registration from simulation CT to online CBCT. In our clinical experience, we do not utilize the deformed target but instead rigidly propagate the target onto the image. The covering physician then makes changes to the target as needed. Initial target contours are created offline with the assistance of abundant clinical information such as diagnostic imaging and surgical pathology reports that are not always readily available when performing online adaptation, and therefore rigidly propagating the initial target volumes helps preserve the initial clinical intent of the prescribing radiation oncologist. In addition, target deformation is variable in quality and may add increased target delineation uncertainty.
To avoid unnecessarily long treatment times, only the portions of OARs that lie in the ContourRing are adjusted in the online adaptive workflow. These are done by an advanced practice radiation therapist to offset the time a radiation oncologist needs to be contouring OARs.15, 22 Due to potential partial contouring of structures and to facilitate more robust template planning, it is often best practice to utilize absolute volume constraints when possible. This will avoid issues caused by partially contoured structures. Following the final approval of the contour changes from the radiation oncologist and the medical physicist, the plan is then reoptimized. It should be noted, in Ethos v1.1, when generating a CTgART plan at the treatment console on the day of treatment, the user can only evaluate the performance of priority 1 and 2 goals.
Patient-specific QA is performed via the MobiusAdapt module of Mobius3D (Varian). Mobius3D analyzes plan DICOM files and performs an independent dose calculation and compares the calculated dose to the dose in the TPS plan.23 Additionally, Mobius3D will perform a 3D gamma analysis. Prior to treatment, an additional verification CBCT is acquired to verify patient positioning. Small rigid shifts can be applied in the event of minor patient movement.23 - 26
Intrafraction Motion Management
Due to the length of adaptive treatment times, intrafraction motion management is an important aspect of CTgART delivery. Our clinic utilizes several different intrafraction motion management techniques driven by site-specific motion management needs. For some abdominal cases, we implement a breath-hold technique that involves the patient holding their breath on end exhale to limit tumor motion.27 A surface guidance system is utilized to monitor breath-hold motion and has been shown to reduce tumor motion to clinically acceptable ranges.26 For sites containing more mobile anatomy, acquisition of a midtreatment CBCT is recommended. Additionally, a post-treatment CBCT may be acquired to assess final patient positioning. To limit breath-holds during treatment, VMAT beam geometry is often preferred due to the reduction in MU ratio compared with IMRT, as illustrated in the Treatment Planning section.
Disease-Site-Specific CTgART Workflow Considerations
Thoracic Malignancies
The role of ART in thoracic malignancies has primarily been explored as an avenue to reduce toxicity for patients receiving SBRT for early stage ultracentral lung malignancies.3, 28, 29 To date, only in silico data have been published on the role of CTgART for patients with ultracentral thoracic malignancies, and all patients treated clinically with early stage ultracentral non-small cell lung cancer with CTgART at our institution are treated with 55 Gy/5 fx on trial (NCT05785845).9 When performing CTgART for thoracic malignancies, the primary image data set for treatment planning is the breath-hold simulation CT scan. Treatment plans are generated from templates where the priority 1 OARs are selected based on the proximity of the target to the OARs. When planning thoracic sites, our clinic will typically utilize arc geometries. Although it has been shown that the CTgART TPS takes longer to optimize VMAT plans compared with IMRT optimization times,8 the decision to utilize VMAT geometries comes from the motivation to minimize the number of monitor units in the plan, the time required to deliver treatment, and patient breath-holds.
Abdominal Malignancies
ART has a role in the treatment of abdominal malignancies as ART allows for interfraction motion management to account for the mobile luminal gastrointestinal tract during abdominal SBRT delivery. There are published experiences on the benefits of ART for patients with pancreatic cancer, liver cancers, and abdominal oligometastases, among other disease processes.30 - 32 Limited clinical data are available on the safety of CTgART for patients with abdominal malignancies, and the results of an ongoing phase 2 trial evaluating CTgART study evaluating 50 Gy/5 fx for patients with pancreatic cancer are eagerly awaited (NCT05764720). In abdominal CTgART planning, the end-exhale breath-hold CT image is typically the primary data set utilized for treatment planning, and patients are treated end-exhale breath-hold if they can tolerate the technique. For patients treated with a free-breathing technique, planning organ at risk volumes (PRV) are derived from CT imaging from the 0 phase (maximal inhalation) and 50 phase (maximal exhalation) data acquired from the 4DCT at the time of simulation, thus creating motion PRVs. For the treatment of abdominal malignancies, arc path is determined based on simulation position. Partial arcs are utilized if the patient is simulated with an arm down, which is sometimes done for patient comfort.
Intrafraction bowel motion is a challenge with abdominal CTgART, particularly for pancreas stereotactic CTgART cases, which can be quite long. Data show that intrafraction bowel motion can cause discrepancies between the dose delivered to OARs as intended with the adapted plan and what is actually treated based on bowel placement at the time of treatment delivery.33, 34 To mitigate this, we often have patients lie in the treatment position for 20 minutes prior to initiating the adaptive process to allow the bowels to settle into a more stable position. A verification CBCT is also acquired prior to treatment delivery, and if bowel has entered the PTV_OPT structure, the treating physician and medical physicist may reinitiate the adaptive contouring and planning process. With regard to contouring within the online adaptive environment, the contour ring is patient-specific and dependent on target size.
Genitourinary Malignancies
The role of ART for genitourinary malignancies is evolving. SMART has an established role in the management of prostate malignancies.35, 36 There is significant intrigue in implementing CTgART workflows for patients with prostate cancer as ART may allow for dose escalation while minimizing rectal and bladder toxicities.37 At our institution, individuals with prostate cancer who receive CTgART are typically unfavorable intermediate- or high-risk patients treated in the study (NCT05628363). Prostate CTgART may be time consuming if the plan requires contouring of all the bowel in the pelvis. Intrafraction bladder filling is also a concern that must be accounted for during treatment planning. Discretion should be used for which portions of the plan require contouring (ie, contour ring extent) as certain OARs may never exceed the provided dose constraints given the lower prescription for elective nodal volumes. The use of IMRT beams is advantageous for prostate CTgART given the shorter optimization time.
Adaptive radiation therapy may also confer significant advantages for patients with bladder cancer as a method of mitigating the deleterious dosimetric effects of changes in bladder filling. Two ongoing studies are evaluating CTgART for patients with bladder cancer (NCT05700227, NCT05295992). When performing CTgART for patients with bladder cancer at our institution, a CT data set acquired with the bladder empty functions as the primary treatment planning data set. Additionally, CT simulation imaging is acquired 30 minutes after the initial CT and is fused to the primary data set. The internal target volume (ITV) margin is constructed such that the ITV structure created from the primary data set also covers the entire bladder in the CT scan acquired after 30 minutes of bladder filling. Treatment planning for the bladder is unique because the organ will change in volume over the course of treatment. Our treatment planning technique for bladder treatments considers the anticipated displacement of OARs away from the target because of bladder filling over the course of treatment by generating OAR minus ITV structures. These structures include the bowel, sigmoid, and rectum minus the ITV and can be used as an assessment of the final dosing to these structures during plan delivery. Adaptive bladder treatment plans can utilize static IMRT beam geometries or VMAT beams; however, static fields are preferred due to the decrease in plan optimization time compared with VMAT plans.
Discussion and Future Directions
Herein we have reviewed in detail our departmental workflow for CTgART as well as specific technical considerations for CTgART workflows in a variety of disease sites. These insights have enabled the development of a robust ART program, treating more than 200 patients with ART per year including 70 patients with CTgART per year.13 This review highlights critical lessons for departments installing their own CTgART programs in hopes of enabling more widespread adoption of CTgART.
The original Ethos CTgART platform had a high-quality CBCT scanner capable of acquiring a scan for contouring over the course of a 17-second breath-hold. The recently upgraded CBCT scanner can obtain a high-quality scan in 6 seconds with an increased field of view and reconstruction algorithm that decreases artifacts from implanted devices, all of which further improve image quality with increased spatial and contrast resolution.21 These improvements provide CBCT images that can rival traditional CT simulation scanners. Critically for the adaptive process, improved imaging quality improves contour delineation and reduces uncertainty. CTgART, and ART in general, is vulnerable to contouring mistakes as adaptive contouring is performed with onboard imaging under time constraints; as such, improved onboard image quality is critical to minimize contouring errors. In addition, with improved onboard imaging hardware and online treatment planning software capable of performing dose calculations on CBCT images,38, 39 CTgART technology may allow for safer and easier implementation of simulation-free treatment workflows.40, 41
Timing studies across numerous disease sites and institutions in both simulated and clinical CTgART suggest that patients remain on the treatment table for 19 to 100 minutes depending on the disease site.13, 42 - 44 Adaptive times outside of the US may be shorter than local experiences due to US requirements for physician plan approval prior to delivering treatment. In Europe, some experiences report therapists and other providers proceeding with the adaptive plan with a post-treatment review by the supervising physician.45 Methods to reduce prolonged treatment times may decrease patient discomfort and reduce the possibility of intrafraction motion that can mitigate the advantages of ART. Possible advancements in the integration of AI-contouring as well as additional optimization of the ART workflow may reduce patient time on the table, resulting in more tolerable treatments and a potential reduction in intrafraction motion of both internal and external patient anatomy. Moreover, further CTgART research may include exploring methods to increase patient comfort during treatment to mitigate discomfort during extended treatment times.
Intrafraction respiratory motion management in stereotactic cases treated on the current CTgART machine platform is limited to breath-hold techniques, whereas traditional linear accelerators have triggered imaging such as automated acquisition of KV x-rays and fluoroscopy. As advanced onboard imaging is combined with traditional C-arm linear accelerator capabilities, it is possible that the optimal interfraction motion management potential of CTgART may intersect with more advanced forms of intrafraction motion management, further reducing uncertainty for high-dose-per-fraction CTgART treatments in the thorax and upper abdomen.
Although a secondary dose calculation algorithm for QA is provided by the vendor, the online ART process has the potential for high-risk errors that cannot be detected by secondary dose calculations alone. For example, 4 out of the top 5 high-risk failure modes identified by Wang et al for CTgART occurred in the online contouring step.46 Therefore, efforts to develop and integrate more sophisticated online QA tools are instrumental in the safe widespread implementation of CTgART. Despite the potential for dose accumulation to enable assessment of dose delivered across adapted plans, our clinical implementation of CTgART does not make use of the Ethos dose accumulation given the vendor recommendations of AAPM TG-132.
Conclusion
CTgART is a powerful tool to improve the therapeutic ratio for radiation therapy but the implementation of CTgART requires a highly standardized workflow. Through the creation of robust treatment planning templates and detailed CTgART processes, it is possible to implement CTgART workflows for a variety of disease sites.
References
Citation
Raranje C, Mueller R, Price AT, Henke LE, Zhao X, Kim H, Laugeman E Schiff JP. Workflow Considerations for Implementing a Cone-Beam CT-Guided Adaptive Radiation Therapy Program. Appl Radiat Oncol. 2024;(4):6 - 16.
doi:10.37549/ARO-D-24-00021
December 1, 2024