Absence of fibrosis after SBRT for HCC in a multiple-transplant patient
Images



CASE SUMMARY
Our patient is a 58-year-old Caucasian woman with a past medical history including two liver transplantations, right kidney transplantation, recurrent hepatocellular carcinoma (HCC) and eradicated hepatitis C infection (HCV). At age 39, the patient had an orthotopic liver transplant (OLT) for HCV cirrhosis. After 11 years (age 50), she developed HCC in the transplanted liver, which was treated with transarterial chemoembolization (TACE). At age 51, the HCC was treated with a second OLT. While on immunosuppression after transplantation, the patient developed calcineurin inhibitor (CNI) toxicity and subsequently end-stage kidney failure that required hemodialysis until she received a deceased donor kidney transplant (DDKT), two years after the second OLT. Six years after her second OLT, she presented to the hospital with nausea, vomiting, and abdominal pain. Her alpha-fetoprotein (AFP) was elevated at 1067 ng/ml and 1902 ng/ml a month later.
IMAGING FINDINGS
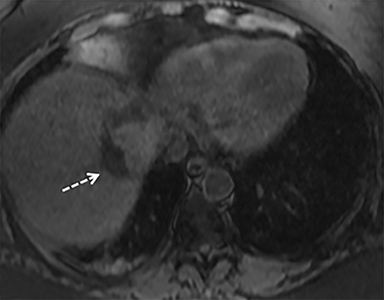
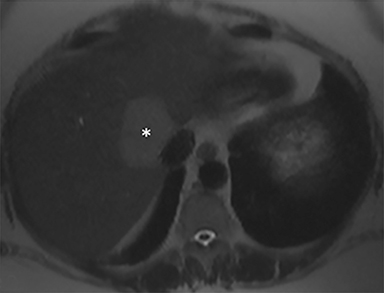
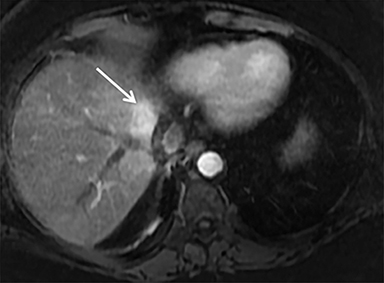
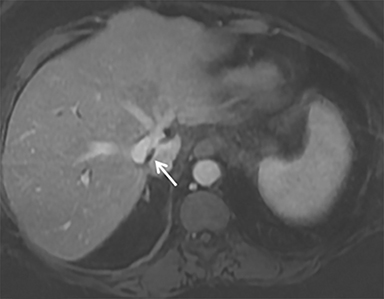
A computed tomography (CT) scan demonstrated a large mass in the dome of the liver (segment VIII) with regions of nodular high attenuation and low-grade enhancement in all contrast phases, suspicious for atypical HCC. Magnetic resonance imaging (MRI) confirmed findings diagnostic of an atypical HCC (Figure 1). Chest CT and bone scans were negative for pulmonary or osseous metastases.
DIAGNOSIS
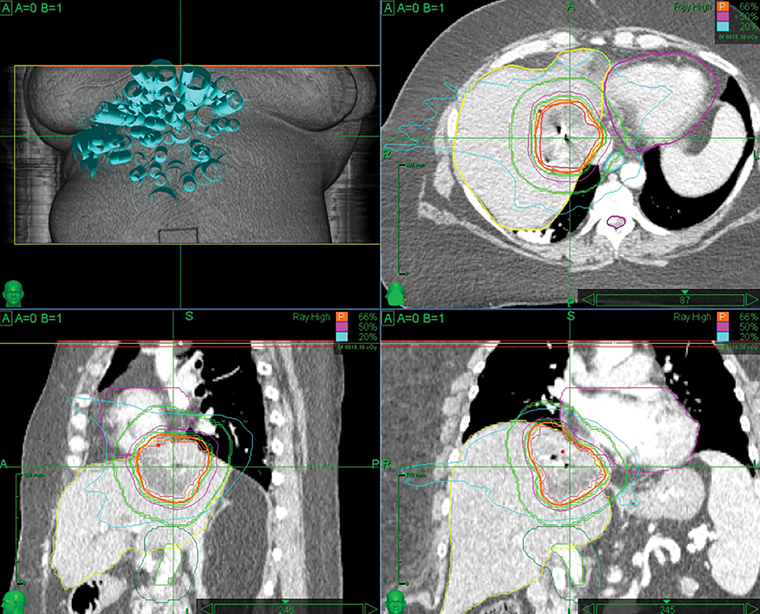
With concern of a new HCC, the patient’s mycophenolate was stopped. Her subsequent immunosuppression regimen consisted of cyclosporine 50 mg BID and prednisone 5 mg once daily. Given that she had had two surgical anastomoses, chemoembolization posed a substantial risk of abscess formation, as well as vascular and chemotherapeutic injuries to the liver. The patient was also not a good surgical candidate due to the liver lesion’s location between the right and middle hepatic veins, producing a future liver remnant that would be too small. The recommendation was a course of stereotactic body radiation therapy (SBRT). Fiducial marker placement was performed using CT guidance. Treatment was performed using the robotic radiosurgery system (Cyberknife, Accuray, Sunnyvale, California), with fiducial tracking. The patient was treated with 5 fractions of 900 cGy, delivered every other day. The prescription isodose line was 66%. The dose was limited by proximity to the heart.
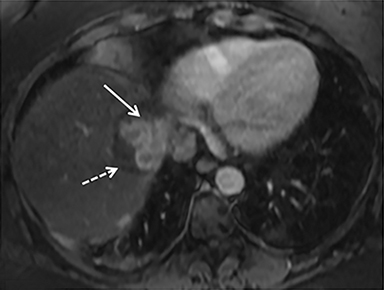
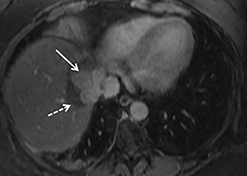
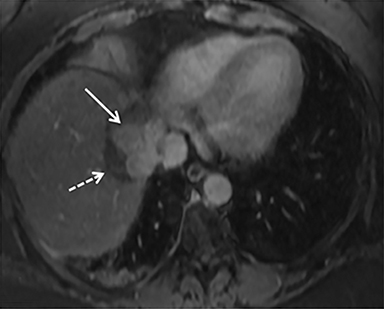
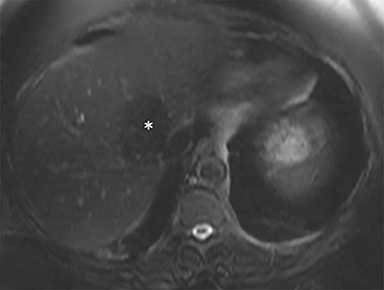
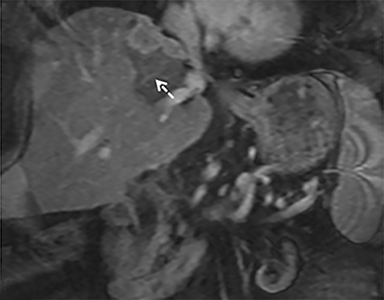
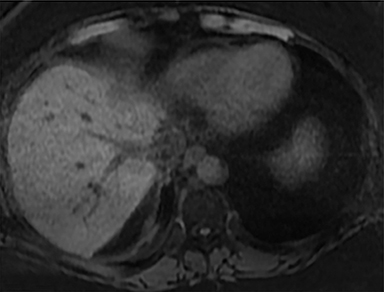
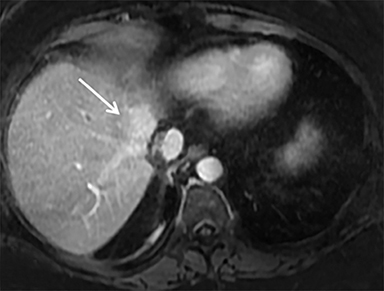
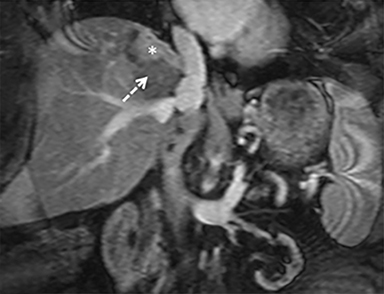
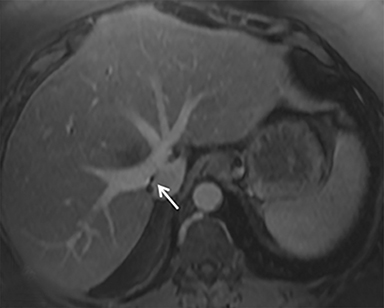
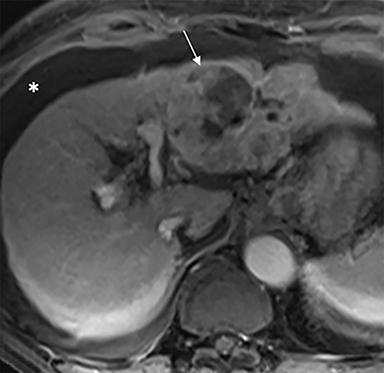
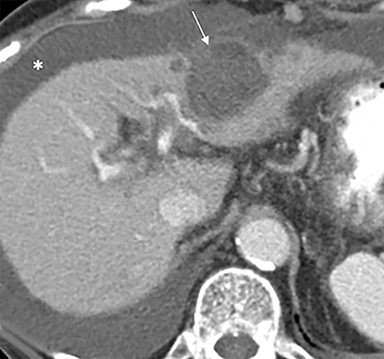
The patient complained of some right upper quadrant pain post-SBRT, likely related to her requirement for common bile duct stent change, as it resolved post change. She also developed symptomatic atrial fibrillation, requiring hospital admission. She had a history of paroxysmal atrial fibrillation for multiple years, and we felt that this was not likely treatment related. The patient’s AFP initially decreased to 325 ng/mL approximately 6 weeks after treatment. It then rose to 450 ng/mL at approximately 6 months post-treatment and 1930 ng/ml at 8 months post-treatment. On follow-up MRI performed 6 months following completion of therapy, the treated mass had decreased substantially (Figure 2). More surprisingly, careful examination of the liver with MRI demonstrated no effects of the radiation treatment (Figure 3). This very unusual lack of treatment effect was striking to the radiologists and the treating clinicians involved in the patient’s care.
DISCUSSION
Pathologically, normal liver tissue treated with SBRT will demonstrate changes like veno-occlusive disease.1 On CT scans and other imaging, local injuries by radiation treatment are commonly seen as density changes in the liver, as well as volume loss. These changes on imaging often manifest as hypodense regions surrounding the target tumor volume. However, visualized density changes do not necessarily correlate with symptomatic toxicity.2
Liver injury (from radiation, for example) initiates a cascade of inflammatory and fibrogenic signals that recruit and transform hepatic stellate cells (HSCs) into myofibroblasts. Myofibroblasts, in turn, lay down connective tissue that leads to fibrosis through the effect of growth factors such as transforming growth factor 𝛽 (TGF-𝛽) released by the injured hepatocytes and macrophages.2,3 There is a dose-dependent increase in TGF-𝛽 in irradiated liver4 and inhibition of this growth factor can help prevent fibrosis. Modulating radiation technique has also been shown to decrease the expression of TGF-𝛽 and improve treatment toxicity in animal models.5 In the case of our patient, she had a good (76% cross-sectional) response to the treatment of a 5.3-×-3.8-cm tumor to 2.5-×-1.9 cm in a relatively short time, with no new density changes on imaging to show radiation treatment and fibrosis. We speculate that her immunosuppressive drugs, cyclosporine and prednisone, may be protecting her from these changes through their effect on TGF-𝛽 expression.
Inflammation regardless of cause (radiation, autoimmune, etc.) promotes fibrosis, and potent anti-inflammatory drugs such as corticosteroids are effective in preventing and treating fibrosis.4 Corticosteroids have been used to treat liver disease and fibrosis—most commonly in autoimmune fibrosis—with improved outcomes. Hepatic fibrosis improves in up to 57% of patients treated with corticosteroids and prevents fibrosis in up to 79% of patients with autoimmune hepatitis.6 In animal models, treatment of HSCs with glucocorticoids reduces the secretion of endogenous TGF-β and TGF-β signaling.7
Cyclosporine is an immunosuppressive drug used as prophylaxis against organ rejection by forming a complex with cyclophilin A (CypA) to inhibit calcineurin.8 Cyclophilin A binding leads to several changes in the cell including immunosuppressive, antitumor, and anti-fibrotic effects. Cyclosporine use in the setting of liver fibrosis from autoimmune hepatitis may help stabilize or reverse fibrosis. 9 TGF-β-mediated fibrosis in the liver occurs similarly to idiopathic pulmonary fibrosis (IPF), where it has been studied more extensively. Cyclosporine has been shown to inhibit TGF-β-mediated fibrosis in IPF by degrading TGF-β-induced-hypoxia inducible factor 1-alpha (HIF-1α), which causes dedifferentiation of myofibroblasts, thus reversing fibrosis.10
CONCLUSION
This is an interesting case of how immunosuppressive drugs—cyclosporine and prednisone—may affect the response of tumor and fibrosis after radiation treatment. Our observation and our research of current literature point to the potential benefits of glucocorticoids and possibly cyclosporine as anti-fibrotic agents.
REFERENCES
- Haddad MM, Merrell KW,Hallemeier CL, et al. Stereotactic body radiation therapy of liver tumors: post-treatment appearances and evaluation of treatment response: a pictorial review. Abdom Radiol. 2016;41(10):2061-2077. doi:10.1007/s00261-016-0768-x.
- Zhang SY, Zhu GY,Li G,Zhang YB,Geng JH. Application of stereotactic body radiation therapy to cancer liver metastasis. Cancer Lett. 2016;379(2),225-229. doi:10.1016/j.canlet.2015.10.029.
- Weigel C, Schmezer P, Plass C, Popanda O. Epigenetics in radiation-induced fibrosis. Oncogene. 2015;34(17):2145-2155. www.nature.com/articles/onc2014145. Accessed April 3, 2018.
- Bansal R, Nagórniewicz B, Prakash J. Clinical advancements in the targeted therapies against liver fibrosis. Mediators Inflamm. 2016;2016: 7629724. doi:10.1155/2016/7629724.
- Meyer JE, Finnberg NK, Chen L, Tissue TGF-ß expression following conventional radiotherapy and pulsed low-dose-rate radiation. Cell Cycle. 16(12):1171-1174. doi:10.1080/15384101.2017.1317418.
- Kim KH, Lee JM, Zhou Y, Harpavat S, Moore DD. Glucocorticoids have opposing effects on liver fibrosis in hepatic stellate and immune cells. Molec Endocrinol. 2016;30(8),905-916. doi:10.1210/me.2016-1029.
- Bolkenius U, Hahn D, Gressner AM, et al. Glucocorticoids decrease the bioavailability of TGF-ß which leads to a reduced TGF-ß signaling in hepatic stellate cells. Biochem Biophys Res Commun. 2004;325(4):1264-1270. doi:10.1016/j.bbrc.2004.10.164.
- Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004; 351(26):2715-2729. doi:10.1056/nejmra 033540.
- Czaja AJ. Review article: the prevention and reversal of hepatic fibrosis in autoimmune hepatitis. Aliment Pharmacol Ther. 2014;39(4):385-406. doi:10.1111/apt.12592.
- Yamazaki R, Kasuya Y, Fujita T, et al. Antifibrotic effects of cyclosporine A on TGF-B treated lung fibroblasts and lungs from bleomycin-treated mice: role of hypoxia-inducible Factor-1a. FASEB J. 2017;31(8)3359-3371. doi:10.1096/fj.201601357r.
