Focused Ultrasound for Ablation in Neurosurgery — Present Use and Future Directions
Images
Abstract
Focused ultrasound (FUS) as a therapeutic modality for the treatment of neurological conditions has seen a rapid expansion over the past decade due to its ability to produce controlled and precise effects noninvasively. FUS has multiple mechanisms of action, but at higher frequencies, thermal ablation is predominant and is capable of precise and controlled lesioning of brain tissue. In particular, transcranial MR-guided focused ultrasound (MRgFUS) surgery has become a well-established tool in functional neurosurgery for movement disorders such as essential tremor and Parkinson’s disease. Since its first US Food and Drug Administration (FDA) approval in 2016, MRgFUS has gained popularity amongst researchers, clinicians, and patients. Ongoing studies to evaluate additional indications are underway. Multiple clinical trials are open for the treatment of psychiatric illness, chronic pain, and epilepsy. Given an aging population as well as the increasing prevalence of diseases treated, the risk-benefit ratio of MRgFUS as a noninvasive ablative therapy should solidify its role as a treatment option for an increasing number of patients.
Keywords: focused ultrasound, high-frequency focused ultrasound, ultrasonic therapy, minimally invasive surgery, clinical device, ablation, brain, essential tremor, Parkinson’s disease, depression
Focused ultrasound (FUS) as a therapeutic modality for the treatment of neurological conditions has seen a rapid expansion over the past decade due to its ability to produce controlled and precise effects noninvasively. In contrast to stereotactic radiosurgery, FUS is capable of nonionizing tissue destruction. This narrative review will focus on its use in thermoablation of brain tissue, though notably FUS is being avidly investigated for applications within neuromodulation as well as transient blood-brain barrier opening.
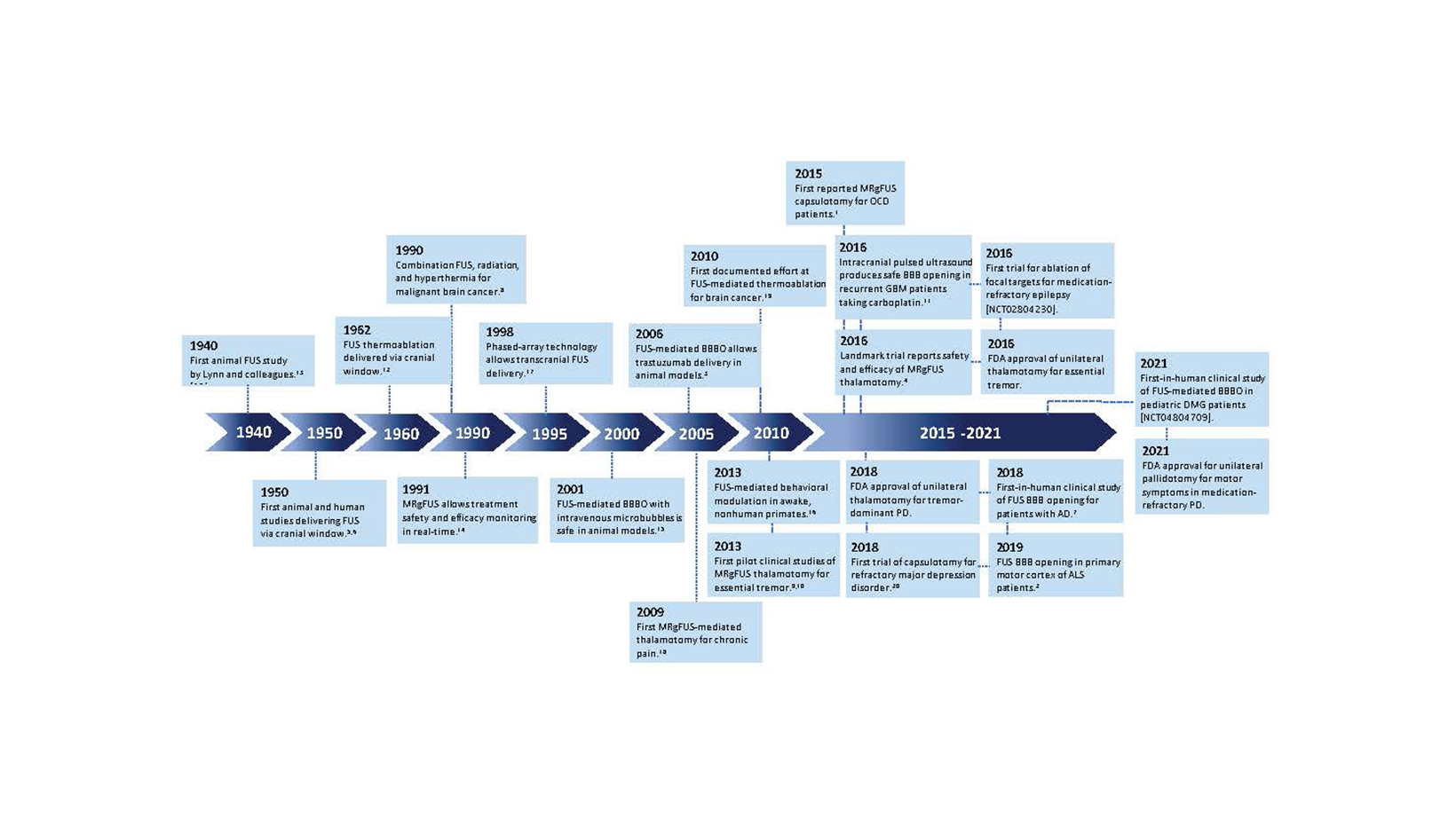
Although ultrasound was discovered in the late 1800s, the invention of FUS is attributed to Johannes Gruetzmacher who placed curved quartz on a piezoelectric generator to concentrate waves. Initial trials in humans targeted deep structures for movement disorders, but lesions were imprecise prior to the advent of modern imaging. Furthermore, large portions of skull were removed to mitigate wave distortion and surface heating. In 1998, the use of MRI and a helmet equipped with 2 arrays and 64 elements was shown to transmit pulsed sonication through a piece of a human skull to induce tissue destruction in an in vivo rabbit brain, which catapulted FUS as a noninvasive modality.
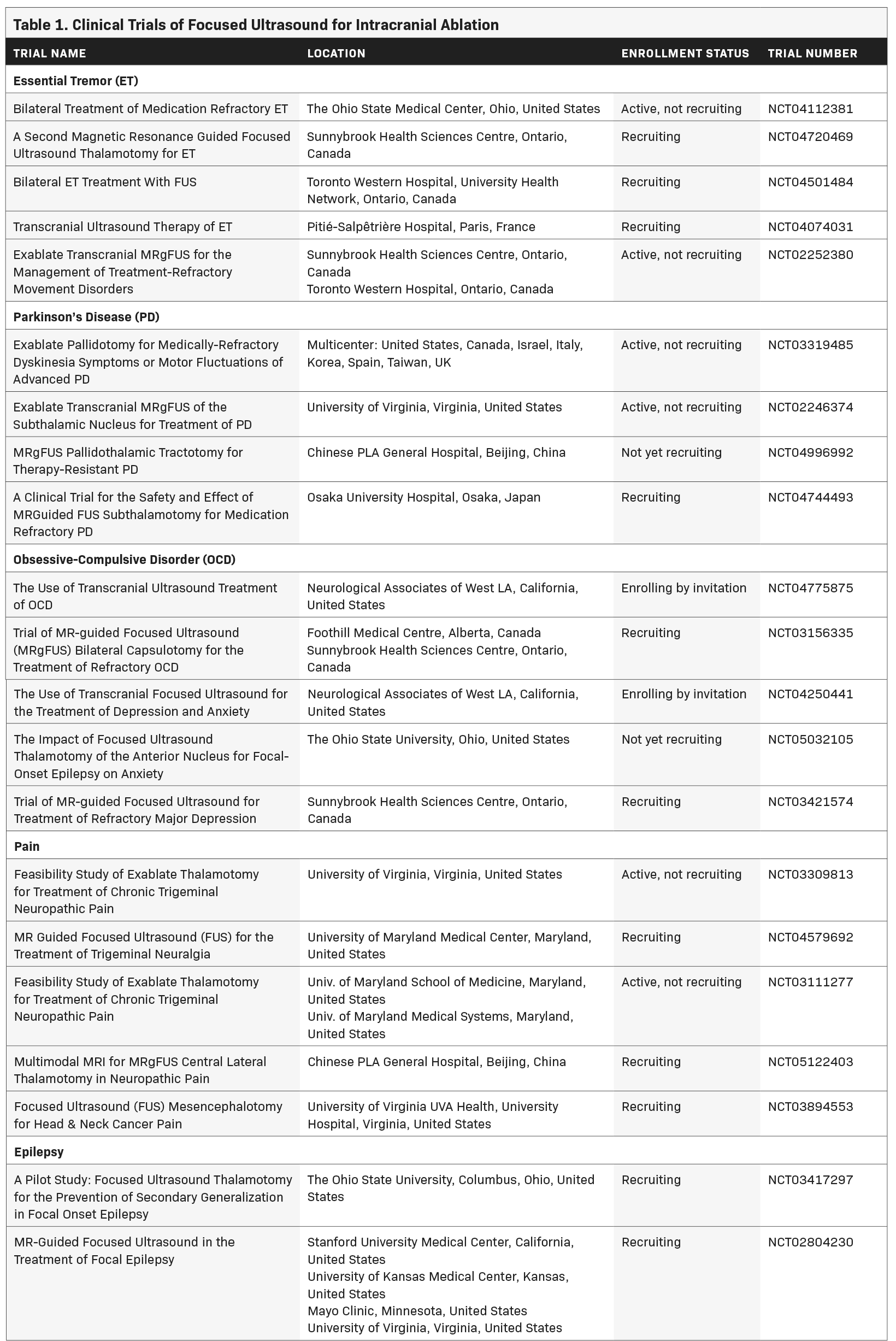
Since that time, myriad developments such as live MRI guidance have improved the safety and efficacy of FUS ablation (Figure 1). As of this writing, 3 neurological indications have been FDA approved for MRI-guided FUS (MRgFUS): thalamotomy for essential tremor (ET), thalamotomy for tremor-dominant Parkinson’s disease (TDPD), and pallidotomy for the motor symptoms of Parkinson’s disease. Multiple additional indications are being investigated in clinical trials as of this writing (Table 1).
Mechanism of Action for Ablation
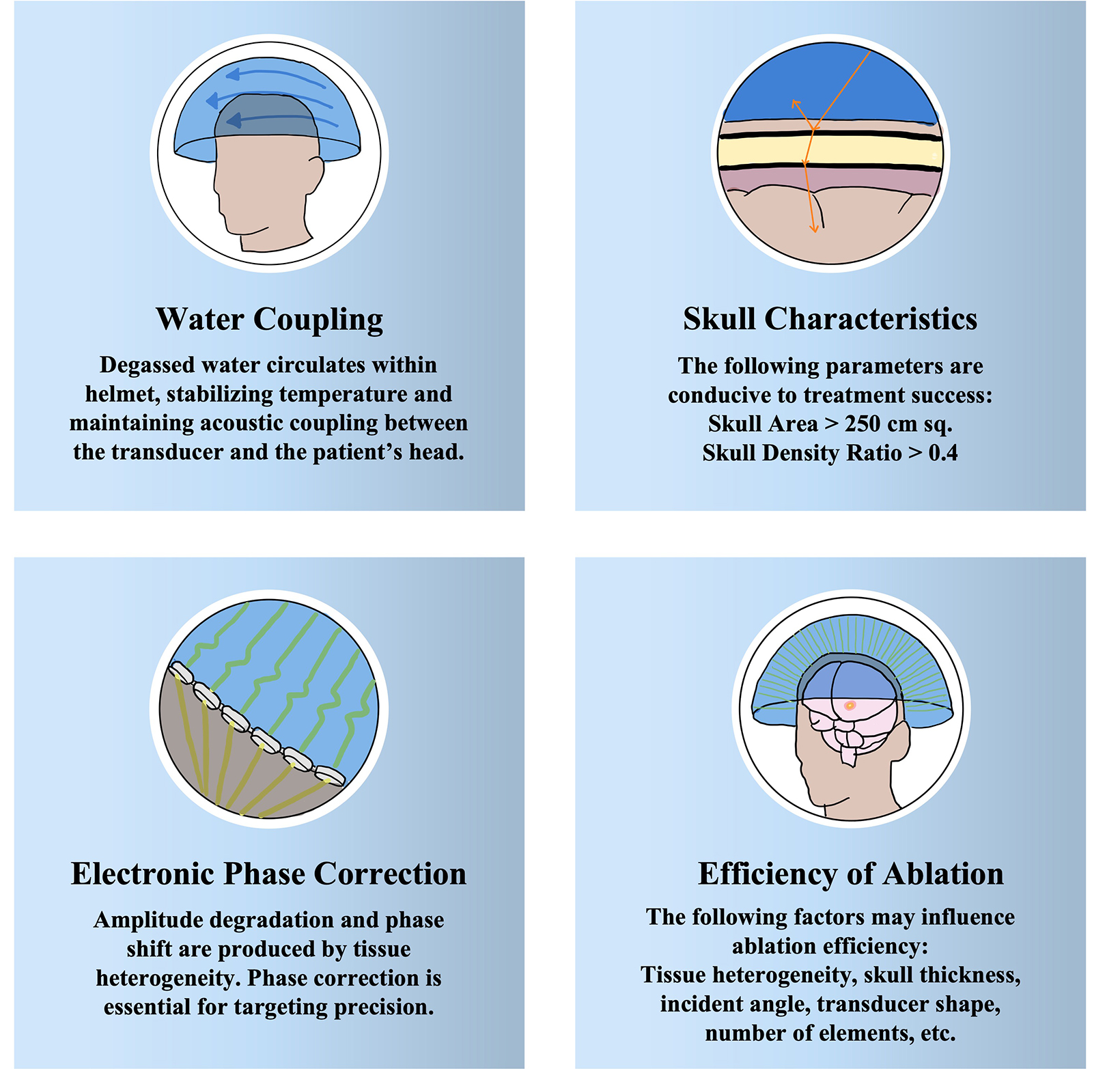
The ablative action of FUS depends on frequency, which leads to either thermal or mechanical tissue destruction. At higher frequencies of 650 kHz, thermal ablation is predominant. The FDA-approved hemispherical transducer (Exablate 4000; Insightec) can achieve peak temperatures of 51°C to 60°C under continuous visual MR guidance and MR thermometry with an accuracy of < 2 mm. Within 48 hours of treatment, 3 concentric zones appear on T2-weighted sequences: 2 inner zones representing necrosis and a zone of perilesional, vasogenic edema, which subsides within 10 days. Several pitfalls should be considered (Figure 2). Higher frequencies and higher incident angles can lead to overheating of the skull due to its high acoustic absorption. High incident angles (> 20°) prohibit targets more proximal to the skull from successful treatment; thermal ablation can only be applied to central brain regions (approximately 3 cm radius around the midcommissural point, the halfway point on a line joining the anterior and posterior commissures).
Thick and poorly homogenous skulls limit the penetration of ultrasound. Preoperative computerized tomography is obtained to assess patient-specific metrics such as skull thickness and skull homogeneity as quantified by skull density ratio (SDR). An SDR below 0.4 is considered inconducive to optimal thermal lesioning and FDA labeling includes only patients with an SDR of 0.4 or higher. At a single-center study in Taiwan, 246 patients were evaluated and 50% had a skull score under 0.4 suggesting that the portion of patients who are ineligible for MRgFUS due to skull characteristics is significant. Air trapped in hair will severely attenuate transmission of ultrasound, thus necessitating a thorough and full shave of the head, a cause of distress in some patients.
Lower frequencies around 220 kHz produce therapeutic mechanical energy by interacting to rapidly expand and contract entrapped gas in a process called cavitation. Cavitation to the point of tissue destruction can be accomplished through a process called histotripsy in which short-duration pulses produce sufficient mechanical action to fragment extracellular matrices and to disintegrate cells into their subcellular constituents. This process is focal and leaves the surrounding tissue intact. Lower frequencies are less susceptible to acoustic absorption and higher incident angles, expanding the potential reach of MRgFUS beyond a 3-cm radius to encompass the entire intracranial space. This remains an area of research but its use intracranially is limited by concerns of an increased risk of hemorrhage in comparison to thermoablation. However, evidence in large animal models suggests there is minimal effect 200 μm from target boundaries and that major hemorrhage and other complications do not occur.
Current FDA-Approved Indications
MRgFUS ablation has become a well-established tool in functional neurosurgery for movement disorders such as ET and Parkinson’s disease. Given the small size of tissue targets, central location within the skull, and an aged patient population with higher operative risk, movement disorders approximate ideal indications for noninvasive thermal ablation.
Essential Tremor
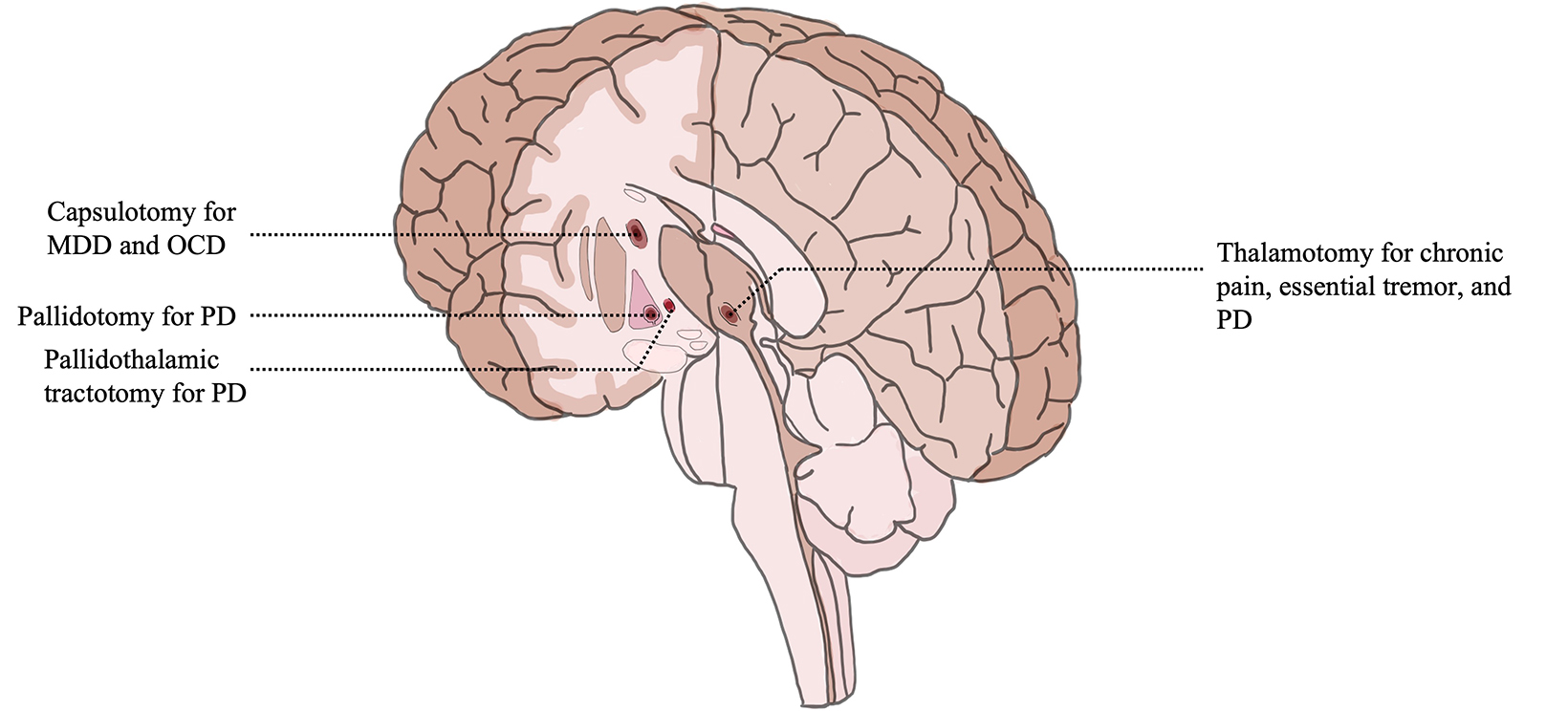
In July 2016, thalamotomy (intentional destruction of thalamic tissue) for refractory ET became the first FDA-approved intracranial use of MRgFUS. ET was once referred to as “benign essential tremor;” however, “benign” was dropped out of consideration for a disease that is often debilitating, involving the hands and arms, and is worse when reaching the target during common daily activities such as holding a glass, eating with utensils, and writing. ET is the most common cause of action tremor in adults and remains a progressive process with no disease-modifying agents. The overall prevalence of ET in 2021 in ages over 65 was 5.67%. In the oldest age groups, median prevalence is 9.3%, with several studies reporting values over 20% without a predilection for gender. Current first-line treatment for ET consists of monotherapy with either propranolol or primidone; however, 30% and 32% of patients see no therapeutic benefit, respectively. Second-line treatments include combination drug therapy of these 2 first-line agents as well as the addition of gabapentin or topiramate. Success rates are generally lower and side effects can be dose limiting. Patients failing adequate trials of at least propranolol and primidone may be offered surgical options, which include treating the ventral intermediate nucleus (VIM) of the thalamus with deep brain stimulation (DBS) or thalamotomy (conventional thalamotomy, Gamma Knife [Elekta], or MRgFUS). A clinical trial randomizing 76 patients with ET to MRgFUS or a sham procedure showed a 47% tremor reduction at 3 months after MRgFUS, which largely persisted after 1 year. Adverse events included gait disturbance in 36% of patients and paresthesias or numbness in 38%, which persisted at 1 year in 9% and 14% of patients, respectively.10 Treatment is largely unilateral due to concerns for increased complications with bilateral thalamotomy, but recent evidence suggests a bilateral staged plan can be safe and effective. A prospective, single-arm, single-blinded phase 2 trial of second-side MRgFUS thalamotomy in 10 patients with ET showed clinically significant improvement in quality of life at 3 months (mean Quality of Life in Essential Tremor score difference, 19.7; 95% CI, 8.0-31.4; P = 0.004). Patients reported they would elect a second-side procedure despite mild adverse effects including 2 with transient gait impairment and a fall, 1 with dysarthria and dysphagia, and 1 with mild dysphagia persisting at 3 months. Currently, ET remains the subject of numerous active clinical trials to expand and optimize its treatment.
Parkinson’s Disease (PD)
PD is the second most common neurodegenerative disease with a steadily increasing global prevalence. More than 6 million individuals are currently affected, which corresponds to a 2.5-fold increase in prevalence over the past generation. This number is projected to double again to over 12 million by 2040 or even as high as 17 million given increasing longevity, declining smoking rates, and increasing industrialization. Tremor due to PD is a rest tremor and typically begins unilaterally, which distinguishes it from ET. Of historical note, 50 patients with PD were amongst the first humans to be treated with FUS in 1960, a procedure that required creation of a skull window and took 14 hours to complete, with temporary improvement at best. As the drug L-dopa was developed, this procedure was understandably abandoned in favor of medical management. It was not until 2018 that thalamotomy for tremor-dominant PD received FDA regulatory approval in the US, becoming the only additional intracranial indication for FUS other than ET. For patients with treatment-resistant PD, DBS has largely replaced conventional lesioning, and targets include the ventral intermediate nucleus, subthalamic nucleus, and internal globus palli- dus, depending on specific patient symptomatology. A randomized, sham-controlled trial of VIM MRgFUS involving 27 tremor-predominant PD patients showed that medication median tremor scores improved 62% in FUS-treated patients compared with 22% after sham procedures; the between-group difference was significant (Wilcoxon P = .04). All adverse events were mild and resolved within 3 months. Initially, transcranial MRgFUS targeting of the subthalamic nucleus was well-tolerated in an open-label pilot study with improvements in motor function; however, a subsequent randomized, sham-controlled trial revealed significant adverse effects including persistent new dyskinesias, motor weakness, and gait and speech disturbances. As a result, efforts to pursue this target have stalled. The internal globus pallidus is commonly targeted in DBS, but its lateral location can be challenging for thermoablation. Nevertheless, MRgFUS pallidotomy in a nonblinded study improved Unified PD Rating Scale part III scores by 39.1% and the Unified Dyskinesia Rating Scale by 42.7% at 12 months, and FDA approval for this location has been granted. The scales measure nonmotor and motor experiences of daily living, patient perceptions, time factors, anatomical distribution, objective impairment, severity, and disability. A promising area of study is lesioning of the pallidothalamic tract for chronic therapy-resistant PD. A recent study of 47 patients resulted in a mean reduction of 84% for tremor, 70% for rigidity, and 73% for distal hypobradykinesia. At present, multiple clinical trials to study thermoablation targets for PD, including international trials, are underway.
Frontier Indications
Psychiatric Diseases
MRgFUS capsulotomy is being studied as a potential treatment for obsessive-compulsive disorder (OCD), depression, and anxiety with small studies published to date. OCD is related to an imbalance of excitatory and inhibitory pathways in the corticostriatal-thalamocortical circuit. Patients are noted to have hyperactive caudates, orbitofrontal cortices, or anterior cingulates. As such, DBS targets have included the ventral striatum, the subthalamic nucleus, the anterior limb of the internal capsule, and the anterior cingulate cortex. MRgFUS treatment has focused on the anterior limb of the internal capsule. Two human trials studied MRgFUS anterior capsulotomy for medically refractory OCD with a mean reduction in the Yale-Brown Obsessive Compulsive Scale of 33% to 37.8% at 2 years in some patients. No serious adverse events were reported. A case report of refractory OCD in the form of constant, debilitating musical obsession achieved durable improvement after MRgFUS capsulotomy. These small studies suggest that the overall response to MRgFUS capsulotomy with respect to symptom response rate and magnitude is comparable to stereotactic radiosurgery capsulotomy, which uses high-dose ionizing radiation. Additional clinical trials to further evaluate OCD and MRgFUS are underway. Major depressive disorder (MDD) is highly prevalent and treatment-refractory in a third of patients and is often comorbid with anxiety and other psychiatric illness. It is a heterogeneous disorder implicating numerous structural and functional brain circuits with historical surgical treatments including the internal capsule, bilateral anterior cingulotomy, subcaudate tractotomy, and limbic leucotomy. In a phase 1 trial of anterior capsulotomy for MDD with MRgFUS, 2 of 6 previously treatment-resistant patients met criteria for response at 6 months (50% reduction in Hamilton Depression Rating Scale) with 4 out of 6 showing no significant change. Clinical trials to further evaluate the efficacy of MRgFUS for MDD and anxiety are underway.
Chronic Pain
The prevalence of pain lasting more than 3 months is as high as 6.9% to 10% in the general population. Extracranial application of FUS was FDA approved in the treatment of pain for bone metastasis in 2012, and clinical trials to investigate intracranial applications of MRgFUS for pain are ongoing. Anterior cingulate, brainstem, spinal cord, and pituitary gland targets have all been discussed in the literature, but the thalamus remains a principal target for ablative therapy given its role in the relay of ascending nociceptive input from neurons of the spinal thalamic tract to key cortical areas. Bilateral central lateral thalamic nuclei thermoablation in 9 patients with chronic neuropathic pain produced pain relief in > 50% at 1 year. A particular area of study is neuropathic pain associated with trigeminal neuralgia, which is being treated with MRgFUS bilateral medial thalamotomy in clinical trial.
Chronic pain is a heterogeneous disease with multifactorial effectors and MRgFUS will not be a cure-all, but will likely join the broad armamentarium of medical and surgical treatment for suffering patients.
Epilepsy
Few case reports have detailed MRgFUS as a treatment in epilepsy patients. One 26-year-old man with gelastic epilepsy and hypothalamic hamartoma received MRgFUS ablation to the boundary area of the lesion to disconnect the hamartoma cells from the base of the hypothalamus. He was able to achieve seizure freedom by decreasing his antiepileptic dosage. One female patient with left temporal lobe epilepsy was treated with 12 sonication sessions to the hippocampus, but failed to achieve the target temperature of (> 54 °C). Nevertheless, at 12 months, seizure frequency had decreased from 3 or 4 seizures per month to near seizure freedom, suggesting either a partial physiological or neuromodulatory effect. Tierney et al performed MRgFUS on 5 patients with benign brain tumors, 3 of whom presented with primary concern for seizure secondary to hypothalamic hamartomas, and for whom thermoablation resulted in 90%, 95% and 100% seizure freedom at 1 year follow-up. Two phase I clinical trials of MRgFUS and epilepsy are ongoing to investigate ablation of the anterior nucleus of the thalamus to prevent secondary generalization in focal onset epilepsy and in patients with comorbid moderate-severe anxiety, respectively. MRgFUS for epilepsy remains a nascent field for continued study.
Conclusion
Since its first FDA approval in 2016, FUS has gained popularity amongst researchers, clinicians and patients as judged by an increase in the number of presentations at international meetings, the volume of publications, and the increase in the number of patients treated. As of 2020, 375,000 patients had received some form of FUS treatment, of which 1% was intracranial. Its utility for noninvasive tissue destruction is particularly relevant in neurological disease where small, deep lesions provide a large effect in a multitude of pathological conditions (Figure 3). FUS is an acoustic, nonionizing therapy and there may be a future role for radiation oncologists in utilizing this treatment. MRgFUS thalamotomy for ET and thalamotomy or pallidotomy for Parkinson’s disease are increasingly utilized by patients and surgeons since regulatory approval in 2016 and 2018, respectively. Initial studies of safety and efficacy for additional indications from depression to trigeminal neuralgia are reassuring and may soon warrant additional regulatory approvals. Use of FUS in neuro-oncology is a nascent and promising frontier. Given an aging population as well as increasing prevalence of diseases considered for treatment, the risk-benefit ratio of MRgFUS as a noninvasive ablative therapy should solidify its role as a treatment option for an increasing number of patients.
References
- Harary M, Segar DJ, Huang KT, et al (2018). Focused ultrasound in neurosurgery: a historical perspective. Neurosurg Focus. 2018;44(2)E2.
- Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol. 1998;24:275-283.
- US Food and Drug Administration. 2022. Exablate Model 4000 Type 1.0 and 1.1 System (“Exablate Neuro”). Accessed September 7, 2022. https://www.fda.gov/medi- cal-devices/recently-approved-devices/exablate-model-4000-type-10-and-11-system-exablate-neuro-p150038s014
- Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66(6):858-861. doi:10.1002/ana.21801
- Elias WJ, Khaled M, Hilliard JD, et al. A magnetic resonance imaging, histological, and dose modeling comparison of focused ultrasound, radiofrequency, and Gamma Knife radiosurgery lesions in swine thalamus. J Neurosurg. 2013;119(2):307-317. doi:10.3171/2013.5.JNS122327
- Chang KW, Rachmilevitch I, Chang WS, et al. Safety and efficacy of magnetic resonance-guided focused ultrasound surgery with autofocusing echo imaging. Front Neurosci. 2021;14:592763. doi:10.3389/fnins.2020.592763
- Tsai KW, Chen JC, Lai HC, et al. The distribution of skull score and skull density ratio in tremor patients for MR-guided focused ultrasound thalamotomy. Front Neurosci. 2021;15:612940. doi:10.3389/fnins.2021.612940
- Raymond SB, Hynynen K. Acoustic transmission losses and field alterations due to human scalp hair. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52(8):1415- 1419. doi:10.1109/tuffc.2005.1509801
- Bader KB, Vlaisavljevich E, Maxwell AD. For whom the bubble grows: physical principles of bubble nucleation and dynamics in histotripsy ultrasound therapy. Ultrasound Med Biol. 2019;45(5):1056-1080. doi:10.1016/j.ultrasmedbio.2018.10.035
- Arvanitis CD, Vykhodtseva N, Jolesz F, Livingstone M, McDannold N. Cavitation-enhanced nonthermal ablation in deep brain targets: feasibility in a large animal model. J Neurosurg. 2016;124(5):1450-1459. doi:10.3171/2015.4.JNS142862
- Louis ED, McCreary M. How common is essential tremor? Update on the worldwide prevalence of essential tremor. Tremor Other Hyperkinet Mov. 2021;11:28. doi: 10.5334/tohm.632.
- Koller WC, Vetere-Overfield B. Acute and chronic effects of propranolol and primidone in essential tremor. Neurology. 1989;39(12):1587-1588. doi:10.1212/wnl.39.12.1587
- Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375(8):730-739. doi:10.1056/NEJMoa1600159
- Iorio-Morin C, Yamamoto K, Sarica C, et al. Bilateral Focused Ultrasound Thalamotomy for Essential Tremor (BEST-FUS Phase 2 Trial). Mov Disord. 2021 Nov;36(11):2653-2662. doi: 10.1002/mds.28716
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019 May;18(5):459-480. doi: 10.1016/S1474-4422(18)30499-X
- Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the Parkinson pandemic. J Parkinsons Dis. 2018;8(s1):S3-S8. doi: 10.3233/JPD-181474
- Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron. 1960;ME-7:166-181. doi:10.1109/ iret-me.1960.5008041
- Sinai A, Nassar M, Sprecher E, Constantinescu M, Zaaroor M, Schlesinger I. Focused ultrasound thalamotomy in tremor dominant Parkinson’s disease: long- term results. J Parkinsons Dis. 2022;12(1):199-206. doi: 10.3233/JPD-212810
- Kleiner-Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006 Jun;21 Suppl 14:S290-304. doi: 10.1002/mds.20962
- Martínez-Fernández R, Rodríguez-Rojas R, Del Álamo M, et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson’s disease: a pilot study. Lancet Neurol. 2018;17(1):54-63. doi:10.1016/S1474-4422(17)30403-9
- Martínez-Fernández R, Rodríguez-Rojas R, Del Álamo M, et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson’s disease: a pilot study. Lancet Neurol. 2018;17(1):54-63. doi:10.1016/S1474-4422(17)30403-9
- Martínez-Fernández R, Máñez-Miró JU, Rodríguez-Rojas R, et al. Randomized trial of focused ultrasound subthalamotomy for Parkinson’s disease. N Engl J Med. 2020;383(26):2501-2513. doi:10.1056/NEJMoa2016311
- Jung NY, Park CK, Kim M, Lee PH, Sohn YH, Chang JW. The efficacy and limits of magnetic resonance-guided focused ultrasound pallidotomy for Parkin- son’s disease: a phase I clinical trial (published online ahead of print, August 1, 2018 ). J Neurosurg. 2018;1-9. doi:10.3171/2018.2.JNS172514
- Health C for D and R. Exablate Model 4000 Type 1.0 and 1.1 System (“Exablate Neuro”) – P150038/S014. FDA. Published online January 3, 2022. https://www. fda.gov/medical-devices/recently-approved-devices/exablate-model-4000-type-10-and-11-system-exablate-neuro-p150038s014
- Gallay MN, Moser D, Rossi F, et al. MRgFUS pallidothalamic tractotomy for chronic therapy-resistant Parkinson’s disease in 51 consecutive patients: single center experience. Front Surg. 2020;6:76. Published January 14, 2020. doi:10.3389/fsurg.2019.00076
- Kim SJ, Roh D, Jung HH, Chang WS, Kim CH, Chang JW. A study of novel bilateral thermal capsulotomy with focused ultrasound for treatment-refractory obsessive-compulsive disorder: 2-year follow-up. J Psychiatry Neurosci. 2018;43(5):327-337. doi:10.1503/jpn.170188
- Davidson B, Hamani C, Rabin JS, et al. Magnetic resonance-guided focused ultrasound capsulotomy for musical obsessions. Biol Psychiatry. 2021;90(10):e49- e50. doi:10.1016/j.biopsych.2020.07.005
- Mustroph ML, Cosgrove GR, Williams ZM. The evolution of modern ablative surgery for the treatment of obsessive-compulsive and major depression disor- ders. Front Integr Neurosci. 2022;16:797533. Published April 6, 2022. doi:10.3389/fnint.2022.797533
- Volpini M, Giacobbe P, Cosgrove GR, Levitt A, Lozano AM, Lipsman N. The history and future of ablative neurosurgery for major depressive disorder. Stereo- tact Funct Neurosurg. 2017;95(4):216-228. doi:10.1159/000478025
- Davidson B, Hamani C, Rabin JS, et al. Magnetic resonance-guided focused ultrasound capsulotomy for refractory obsessive compulsive disorder and major depressive disorder: clinical and imaging results from two phase I trials. Mol Psychiatry. 2020;25(9):1946-1957. doi:10.1038/s41380-020-0737-1
- di Biase L, Falato E, Caminiti ML, Pecoraro PM, Narducci F, Di Lazzaro V. Focused ultrasound (FUS) for chronic pain management: approved and potential applications. Neurol Res Int. 2021;2021:8438498. Published 2021 Jun 29. doi:10.1155/2021/8438498
- Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66(6):858-861. doi:10.1002/ana.21801
- Insightec. A feasibility study of focused ultrasound to perform bilateral medial thalamotomy for the treatment of chronic trigeminal neuropathic pain. clinicaltrials.gov. Published August 10, 2021. Accessed August 19, 2022. https://www.clinicaltrials.gov/ct2/show/NCT03309813
- Lescrauwaet E, Vonck K, Sprengers M, et al. Recent advances in the use of focused ultrasound as a treatment for epilepsy. Front Neurosci. 2022;16:886584. Published 2022 Jun 20. doi:10.3389/fnins.2022.886584
- Yamaguchi T, Hori T, Hori H, et al. Magnetic resonance-guided focused ultrasound ablation of hypothalamic hamartoma as a disconnection surgery: a case report. Acta Neurochir (Wien). 2020;162(10):2513-2517. doi:10.1007/s00701-020-04468-6
- Abe K, Yamaguchi T, Hori H, et al. Magnetic resonance-guided focused ultrasound for mesial temporal lobe epilepsy: a case report. BMC Neurol. 2020 Apr 29;20(1):160. doi:10.1186/s12883-020-01744-x
- Tierney TS, Alavian KN, Altman N, et al. Initial experience with magnetic resonance-guided focused ultrasound stereotactic surgery for central brain lesions in young adults (published online ahead of print, January 14, 2022). Neurosurg. 2022;1-8. doi:10.3171/2021.10.JNS21416
- State of the Technology. Focused Ultrasound Foundation. Published May 4, 2022. Accessed August 18, 2022. https://www.fusfoundation.org/the-technology/ state-of-the-technology/
Citation
Yoh N, Tazhibi M, Englander ZK, Wu CC, Baltuch G. Focused Ultrasound for Ablation in Neurosurgery — Present Use and Future Directions. Appl Radiat Oncol. 2022;(4):14-22.
December 23, 2022
