Glioblastoma: Multidisciplinary treatment approaches
Images
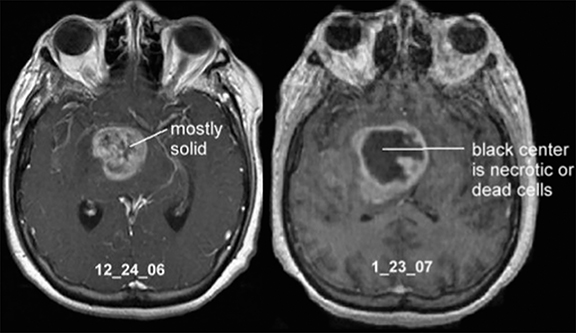
Glioblastoma, also known as glioblastoma multiforme (GBM), is the most common and most malignant primary tumor of the central nervous system (CNS) (Figure 1). According to the Central Brain Tumor Registry of the United States (CBTRUS) 2007-2011, 52.751 of 343.171 brain tumors (15.37%) were GBM, representing 45.6% of all malignant primary brain tumors.1
During the early 19th century, glioblastoma was considered GBM of mesenchymal origin and was defined as a sarcoma. In 1863, Rudolf Virchow demonstrated its glial origin,2 and in 1914 Mallory proposed the term glioblastoma multiforme. However, it was not until 1925 that Globus and Strass presented a complete description of the neoplasm, at which point the most common term became spongioblastoma multiforme. Finally, in 1926, Bailey and Cushing successfully reintroduced the term originally proposed by Mallory: glioblastoma multiforme.
There are two types of GBM, each distinguished by origin and molecular phenotype: primary, which represents the majority of GBM patients and develops rapidly over the course of several weeks; and secondary, which presents as lower-grade gliomas and eventually progresses to grade IV. Once a patient is diagnosed with GBM, the overall median survival time for those treated with the Stupp scheme is approximately 15 months.3
Technology
Treatment protocols for GBM combine surgery followed by concurrent radiation therapy with temozolamide and adjuvant temozolamide (TMZ). These approaches provide palliation and moderate survival benefit.3-5
Clinical Applications
Surgery
In multidisciplinary regimens, glioma resection remains the mainstay given its central role in establishing a histologic diagnosis and in relieving symptoms of mass effect by mechanical cytoreduction. The objective is to provide maximal tumor resection with preservation or restoration of neurologic function.6,7 Unfortunately, patients nearly always experience tumor recurrence, as these tumors invade and infiltrate surrounding normal tissue, making curative resection unlikely.
Advanced Surgical Techniques
The best established technique for assessing the eloquent cortex to guide resection is direct cortical stimulation (DCS).8,9 With this approach, low-current stimulation of the brain creates a transient localized lesion, and testing of language function during DCS can help assess the site of importance in language function. The mapping of motor and language areas of the brain has allowed for more aggressive resections of high-grade gliomas by minimizing the risk of potential deficits.
In fluorescence-guided resections, 5 aminolevulinic acid (5-ALA) is used as an orally administered prodrug, which is metabolized intracellularly to protoporphyrin IX and emits a red-violet fluorescent signal evidenced by blue light. This agent accumulates in certain tumor types and, thus, can help differentiate tumor from normal surrounding brain tissue.10
Image-guided surgical techniques have helped safely assist the extent of surgery in eloquent cortical areas where resection is frequently abandoned before gross total resection to avoid neurologic deficits. This is the reason for neuro-navigation based on preoperative functional MRI (fMRI), the most common noninvasive tool that can provide additional information on the anatomical relationship between borders of the tumor, specifically infiltrating tumors and eloquent areas.11-14 Motor mapping can be performed either with the patient awake or under general anesthesia, while speech mapping requires the use of an awake anesthesia technique, at least during the mapping portion. Concomitant with neuronal activity is an increase of blood flow through local cerebral vessels. These changes in cerebral blood flow can be visualized by a method of fMRI that measures variations in the area of interest that are dependent on blood oxygen level.
Chemoradiotherapy
After surgery, chemoradiotherapy is considered the standard treatment. During the delineation and planning of radiotherapy treatment, the radiation oncology team uses acronyms like GTV (gross tumor volume), CTV (clinical target volume) and PTV (planning target volume). The doses and treatment phases are based on protocols determined by the European Organization for Research and Treatment of Cancer (EORTC) and the Radiation Therapy Oncology Group (RTOG). Based on the RTOG guidelines, the initial volumes (T2/FLAIR + gross/residual tumor plus resection cavity) receive 4600 cGy/23 fractions followed by a boost to 1400 cGy/7 fractions to gross/residual tumor plus resection cavity. In a study by Kelly et al,15 the isolated tumor cells were noted to extend to cover T2 changes and beyond on MRI, which was confirmed with serial stereotactic biopsies; this is the reason for the definition of the initial GTV treated to lower doses (eg, 46 Gy). These PTV are based on the 1980 study by Hochberg and Pruitt16 that showed, using computed tomography (CT), 78% of recurrences were within 2 cm of the margin of the initial tumor bed, and 58% were within 1 cm. This pattern was validated by Wallner et al.17 These data are the basis for the definition of the boost to GTV treated to higher doses (eg, 60 Gy). According to the EORTC, only a treatment volume receives 60 Gy in 30 fractions. The GTV corresponds to the surgical resection cavity plus any residual enhancing tumor (postcontrast T1-weighted MRI scans); the CTV comprises the GTV plus a margin of 20 mm; and finally, PTV is equal to CTV plus a margin of 3-5 mm.
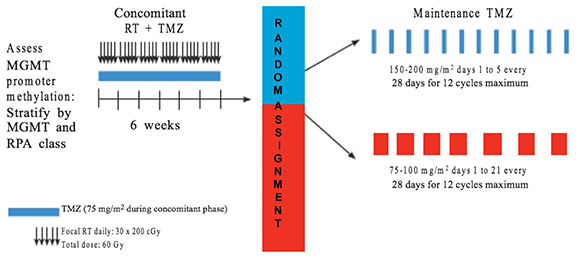
Better results have been obtained with a combination of RT and temozolamide (TMZ), with standard dosing for concomitant TMZ therapy being 75 mg/m2/d given daily during radiation therapy (RT) followed by 150-200 mg/m2/d for 5 days every 28 days for a total of 6 cycles.3 The RTOG-0525, which consisted of 833 patients, did not show a statistically significant difference between a conventional TMZ regimen and a dose-dense TMZ protocol. The overall survival (OS) was 16.6 vs. 14.9 months, and progression-free survival (PFS) was 5.5 vs. 6.7 months, respectively. The dose-dense protocol increased grade 3 toxicities from 34% to 53% (Figure 2).
In an attempt to shorten treatment duration in older patients, hypofractionated radiation therapy (HFRT), which gives a higher radiation dose per fraction in fewer total fractions over a shorter period (eg, 40 Gy in 15 fractions over 3 weeks), has been shown to be equivalent in older patients to the standard of 60 Gy in 30 fractions over 6 weeks.18
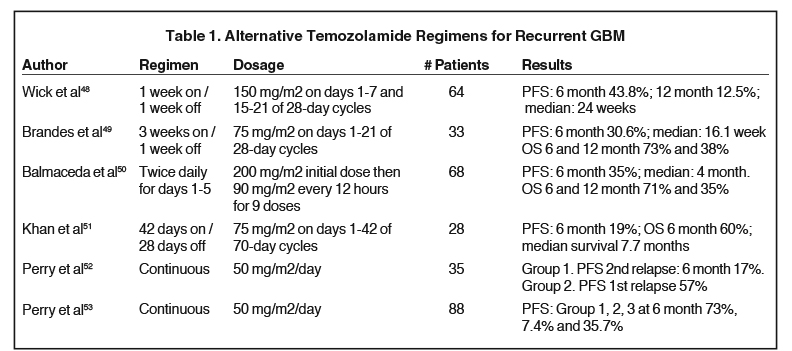
Stereotactic radiosurgery (SRS) has been used as a boost after conventional treatment or in cases of recurrence.19,20 Some authors theorize that SRS could be useful as a local radiation boost to the “worst” part of the tumor, which could be identified with MR perfusion imaging, or in areas with the highest creatine to coline ratio on MR spectrocospy; however, some publications have shown no benefits21,22 (Figure 3). There is no level I evidence that supports the addition of SRS as an initial treatment. Level II evidence suggests a modest survival benefit after SRS in selected patients; on the other hand, attempts to deliver a higher cumulative dose of 70.4 Gy using hyperfractionation schemes also failed to show a survival advantage.23 With the implementation of TMZ into standard GBM therapy, the role of SRS in both newly diagnosed and recurrent GBM continues to be investigated. Clinical oncologists should consider different schemes (Table 1) in treatment regimens with TMZ (recurrence), which can be monitored closely, considering the advances in imaging techniques, localization, chemotherapy (CHT), biological agents and radiosensitizers.
Chemotherapy
CHT includes alkylating agents, nitrosoureas, procarbazine, topoisomerase inhibitors, platinoids, vincristine, and estrogen receptor antagonists.24-32 Before TMZ therapy, the role of CHT in GBM was controversial. A meta-analysis of 12 randomized trials (> 3000 patients) showed an increase in 1-year survival from 40% to 46% with CHT.33 TMZ is an alkylating agent stable only at acidic pH.34 This prodrug undergoes rapid chemical conversion in the systemic circulation at physiological pH to the active compound, which will react with water. This results in an unstable cation, which transfers a methyl group to the DNA, causing the cytotoxic effect of temozolomide because it depletes the DNA-repair enzyme O6-methylguanine-DNA methyltransferase (MGMT). In 2009, bevacizumab, an anti-VEGF inhibitor, was approved for the treatment of recurrent glioblastoma. It has been administrated as a single agent or in combination with cytotoxic therapy; however, neither regimen has been shown to prolong OS.
Molecular Diagnostics
Molecular diagnostics are important because low levels of MGMT in tumor tissue are associated with longer survival among patients with GBM.35-36 Approximately 45% of patients with newly diagnosed GBM have methylation of the MGMT promoter that responds better to TMZ.37
Recently, a paper by Parsons and colleagues38 demonstrated the existence of a glioma-associated mutation in isocitrate dehydrogenase-1 (IDH1) in 12% of patients with GBM. IDH is an enzyme involved in oxidative metabolism.39 Mutations in IDH1 were associated with younger age, secondary GBMs (grade IV tumors that arise from biopsy-proven, lower-grade predecessors), and increased OS. IDH1 mutations have been found more frequently in secondary GBM (sGBM) compared with primary GBM (pGBM); patients with GBM with IDH1 mutations have improved survival (45.6 vs 13.2 months).40,41 Additionally, Sanson and colleagues42 found improved progression-free survival (PFS) of 55 months in patients with IDH1 mutation vs 8.8 months in those without mutation. Secondary GBM is characterized by IDH1, TP53, and ATRX mutations, while primary GBM frequently show molecular alterations in EGFR, PDGFRA, PTEN, TP53, NF1, and CDKN2A/B, as well as TERT promoter mutations, but not IDH mutations.
Another molecular prognosticator is alpha thalassemia/mental retardation syndrome X-linked (ATRX), a gene that produces a protein involved in chromatin remodeling. Jiao et al43 showed that ATRX mutations appear in 57% of patients with secondary GBM, and are rare in primary GBM (4%), noting that nearly half of adult-infiltrating gliomas that harbored an ATRX mutation also contained an IDH1 mutation.44
Electrical Fields
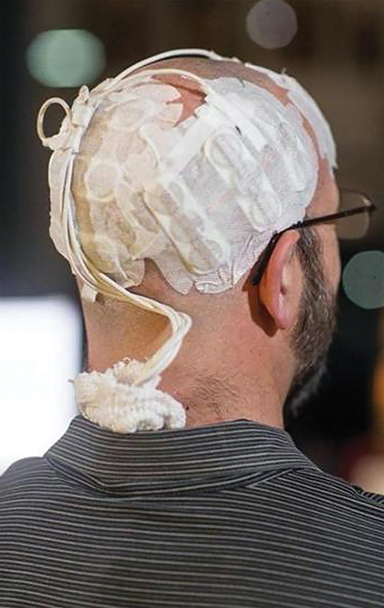
Tumor-treating fields (TTF) are low- intensity, medium-frequency, alternating electric fields administered using insulated electrodes on the skin surrounding the region of a malignant tumor (Figure 4). This disrupts cancer cell mitosis. TTF selectively affects dividing cells while quiescent cells are left intact, acting in 2 modes: arrest of cell proliferation and destruction of cells while undergoing division.45
In 2011, the NovoTTF-100A system (Novocure Ltd., Haifa, Israel) was approved by the U.S. Food and Drug Administration for treating recurrent glioblastoma. While a phase 3 clinical trial comparing stand-alone TTF with TMZ for recurrent glioblastoma failed to demonstrate a significant difference in OS between both groups,46 it is important to mention that a comparative subgroup analysis of the original trial demonstrated that TTF accounted for a proportion of the responders to treatment than the conventional CHT group, with a median response duration of 7.3 vs 5.6 months.47 At interim analysis, the EF-14 Trial,117 which enrolled 700 patients from the United States, Europe, South Korea, and Israel, showed that 315 patients who received TMZ and treatment with the NovoTTF-100A system (now called Optune) survived an average of 19.6 months vs. 16.6 months for those receiving only TMZ. Additionally, patients treated with Optune had an increased PFS of 3 months compared to those who did not (7.1 vs 4.0 months). The OS at 2 years was 43% with Optune and TMZ, and 29% with TMZ alone.This phase 3 clinical trial was terminated at interim analysis due to early success, and was presented at the Society of Neuro-Oncology (SNO) 2014 Annual Meeting in Miami, Florida, by Dr. Roger Stupp.
Toxicity
The presence of neurological deficits following neurosurgery is declining, thanks to advances in tumor localization and delineation, functional imaging, and operative techniques. Despite these advances, some tumor localizations remain a common cause of cranial nerve injury.
Common radiation-induced adverse effects include: fatigue, anorexia, alopecia, erythema of the scalp, serous otitis, nausea, vomiting, exacerbation of neurologic deficits, headaches and seizures. Considering the poor prognosis of these patients, reports of long-term complications in high-grade gliomas (other than radiation necrosis) are rare.
CHT is generally neurotoxic,54 but the CNS is protected when the blood-brain barrier is intact. Therefore, signs of encephalopathy such as headaches, altered cognition, or arousal with or without seizures are rare after systemic administration of conventional CHT doses. The use of glucocorticosteroids,55 opioids and antiepileptics may result in behavioral and mental changes, anxiety, nervousness, insomnia, or euphoria. The toxicity caused by TTF is low and consists mainly of skin reactions at the site of the electrodes.
Diagnosis of Recurrence
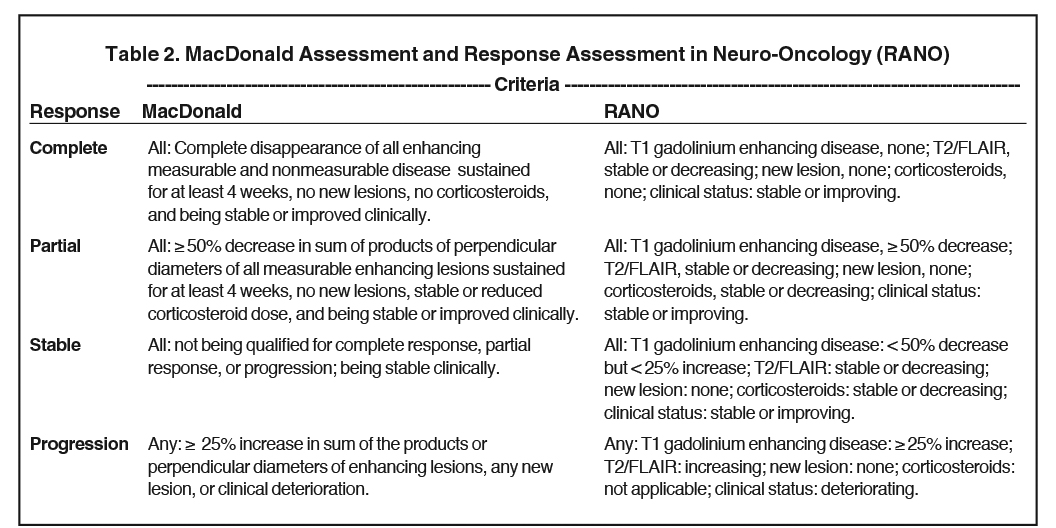
Tumor recurrence occurs in almost all patients, and standards of care are incompletely defined in recurrent or progressive glioblastoma. All therapeutic modalities mentioned above can be used again, modified as needed with each case. However, one should note that the appearance of enhancing lesions on MR imaging within the first 6 months after completing chemoradiation therapy poses a challenge as it can reflect true progression (TP) or treatment-related changes known as pseudoprogression (PSP). Criteria for response and progression in GBM should be discussed 3 to 6 months after completing chemoradiation, as many patients show increased contrast enhancement and T2/FLAIR hyperintensity in the radiation treatment field. As a result, MR imaging every 3 months remains the gold standard for diagnosing response or progression in GBM. Given the uncertainty of PSP and TP, it is important to consider criteria such as the MacDonald criteria and RANO criteria (Table 2). MacDonald criteria does not take PSP into account when defining disease progression, whereas the more contemporary RANO criteria defines progression as the development of a new area of enhancement outside of the prior radiation field at < 12 weeks after completion of chemoradiotherapy, confirmed by biopsy or clinical decline. Currently, the best standardized tool for evaluating response or progression is the RANO criteria.
Conventional MRI, such T1-weighted, gadolinium-enhanced (T1-Gad); T2-weighted; or fluid-attenuated inversion recovery (FLAIR) sequences, do not differentiate recurrent tumors from radiation injury. Advanced MRI techniques such as MR spectroscopy (MRS), perfusion-weighted imaging (PWI), and diffusion-weighted imaging (DWI); and biological imaging such as positron emission tomography (PET), have shown promise in differentiating glioma recurrence or progression from treatment changes.56 Several studies evaluating the use of either MR spectroscopy or MR perfusion found that relative cerebral blood volume (rCBV),57-61 as well as Cho/Cr and Cho/NAA ratios,62-68 are good predictors of recurrent tumor. The Cho/NAA and NAA/Cr ratios44 are good for differentiating tumor recurrence from radiation necrosis, and higher Cho/NAA ratios were associated with a greater probability of tumor infiltration and recurrence.41,45 With PET techniques, imaging with radiolabeled amino acids offers a powerful approach for noninvasive evaluation of brain tumors. Recent studies demonstrated that [11 C]-methionine (MET), O-2-[18 F]-fluoroethyl-L -thyrosine (FET), as well as 3,4-dihydroxy-6-[18 F]-fluoro-L-phenyl-alanine (FDOPA) could be good techniques for detecting glioma recurrence and complementing MRI.69-77 Amino acid PET can detect a metabolically active tumor, and this amino acid uptake in patients with suspected glioma recurrence may be useful in guiding new treatment options to optimize effects in patients with recurrent malignant GBM.
Treatment Options for Tumor Recurrence
The option of repeating surgery in patients with progressive or recurrent glioblastoma remains controversial. Some retrospective studies proposed a survival benefit after reoperation78-81 taking into account age, Karnofsky (KPS) and Eastern Cooperative Oncology Group (ECOG) scales, MGMT promotor methylation, tumor volume, localization, extent of resection, ependymal involvement and tumor in noneloquent areas, while others did not.82-84 Ringel et al85 assessed 503 patients undergoing 1 to 4 re-resections for recurrent GBM with a median OS of 25.0 months after initial surgical treatment, and 11.9 months after first re-resection.
Re-irradiation is a similarly controversial option for patients with recurrent glioblastoma; total doses between 30-36 Gy in 2-3.5 Gy fractions with or without intensity modulation have been used.86,87 In an attempt to retreat larger volumes of recurrent disease with higher doses, the departments of human oncology, medical physics, and biostatistics at the University of Wisconsin, explored pulsed reduced-dose-rate radiation therapy (PRDR), in which the dose-rate effect is most dramatic between 0.01 and 1 Gy/min compared to conventional radiation therapy, in which a dose of 2 Gy is delivered at a dose rate of 4-6 Gy/min. The Wisconsin reirradiation experience consisted of PRDR in a series of 0.2-Gy pulses separated by 3-min intervals, creating a dose rate of 0.0667 Gy/min, reducing the linac dose rate to 1 Gy/min during each 0.2-Gy pulse, which would enhance the therapeutic ratio, taking advantage of the sublethal damage repair of normal tissue and the phenomenon known as low-dose hyper-radiosensitivity (LDHRS) of the tumor.118 On the other hand, SRS can be considered in patients with small volume and well-defined disease.88 Given that GBM recurrences are predominantly local, proponents of using SRS note that it allows for dose escalation with a rapid fall-off of gradient doses limiting exposure to organs at risk (OARs). Skeptics report that GBM is a highly infiltrative disease that extends beyond the apparent margins, making the use of a highly conformal technique inadvisable. In 2014, Larson et al reviewed the literature and found 9 studies describing the use of Gamma Knife (Leksell Gamma Knife; Elekta, Stockholm, Sweden) radiosurgery for recurrent GBM,89 with a median OS range of 9-17.9 months from salvage SRS, and a median progression-free survival (PFS) range of 4.6-14.9 months.90-98
Beyond chemotherapy with alkylating agents (TMZ or nitrosoureas), other classical non-alkylating chemotherapeutics have been studied, including carboplatin (CABARET trial) and irinotecan (BRAIN trial). Evaluated in randomized phase 2 trials as add-ons to bevacizumab,99,100 these agents showed no difference in outcome, and caused additional toxicity.
Another therapeutic option to consider is an intravenous humanized anti-VEGF monoclonal antibody that impairs angiogenesis by targeteing the VEGF ligand (bevacizumab). The induction of VEGF by ionizing radiation enhances blood vessel protection and, subsequently, tumor resistance. Anti-VEGF therapies block this protection, and enhance the effect of therapeutic radiation,101,102 but the future role of bevacizumab is uncertain since the EORTC 26101 trial failed to demonstrate superiority for OS of lomustine plus bevacizumab over lomustine alone.103
Extracranial Metastatic Disease
The first case of extracranial metastasis was reported by Davis in 1928,104 with a GBM disseminated to the lung, chest wall and soft tissue of an arm. Extracranial metastasis is a unique but rare manifestation of GBM reported in < 2% of cases,105-112 with only 83 cases published between 1928 and 2009. This rarity is related to patients’ short period of life, with a median OS of 10.5 months, a median time from symptom onset to diagnosis of primary GBM of 2.5 months, a diagnosis to extracranial metastasis detection time of 8.5 months, and metastasis to death time of 1.5 months.113,114 The infrequency of this extracranial demonstration is perhaps due to intrinsic biological obstacles that prevent tumor GBM cells from infiltrating and surviving beyond the neural environment, such as the blood–brain barrier, absence of a lymphatic system within the brain and spinal cord to allow systemic dissemination, thickened basement membrane of blood vessels, and thickened dura mater around intracranial veins that prevents tumor cell penetration.
Conclusion
In general, overall survival of GBM patients has improved little over time, despite advances in molecular diagnostics, neurosurgery, radiation therapy, chemotherapies, imaging techniques, and immunotherapy, and continues to pose a difficult challenge for patients, family and clinicians. Life expectancy in patients with unmethylated MGMT is 14.8% and 8.3% at 2 and 5 years, respectively, vs. 48.9% and 13.8% in those with MGMT promoter methylation.115 In 2009, the randomized phase 3 study of a 5-year analysis of the EORTC-NCIC trial119 showed that OS in 573 patients was 27.2%, 16.0%, 12.1% and 9.8% at 2, 3, 4 and 5 years, respectively, with radiotherapy and TMZ, vs. 10.9%, 4.4%, 3.0% and 1.9% with radiotherapy alone. The methylation of the MGMT promoter was the strongest predictor of results with TMZ. Research must continue to guide treatment based on current developments, taking into account prognostic factors to offer patients a greater quantity and/or quality of life.
While standard treatment for intracranial GBM is surgical resection followed by concurrent radiotherapy and chemotherapy,116 treatment strategies for metastatic disease are sparse, and optimal treatment has not been determined. Clinical trials120 are attempting to establish the most appropriate therapy for recurrent GBM. In the case of metastatic lesions, it would be an interesting option to recruit patients for clinical trials to establish the most promising treatment; however, the rarity of this condition and its prognosis would hamper success.
References
- Ostrom Q, Gittleman H, Liao P, et al. CBTRUS statical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014; 16 Suppl 4:iv1-iv63.
- Virchow R. Pathologie In Die Krankhaften Geschwülste. Berlin. 1864-1865.
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
- Davis ME, Stoiber AM. Glioblastoma multiforme: enhancing survival and quality of life. Clin J Oncol Nurs. 2011;15:291-297.
- Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842-1850.
- Chaichana K, Zadnik P, Weingart J, et al. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg. 2013;118(4):812-820.
- Chaichana K, Cabrera-Aldana E, Jusue-Torres I. When gross total resection of a glioblastoma is possible, how much resection should be achieved? World Neurosurg. 2014;82(1-2):e257-265.
- De Witt Hamer PC, Robles SG, Zwinderman, et al. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a metaanalysis. J Clin Oncol. 2012;30(20):2559-2565.
- Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 208;358(1):18-27.
- Tykocki T, Michalik R, Bonicki W, Nauman P. Fluorescence-guided resection of primary and recurrent malignant gliomas with 5-aminolevulinic acid. Preliminary results. Neurol and Neurochir Pol. 2012;46(1):47-51.
- Hirsch J, Ruge MI, Kim KH, et al. An integrated functional magnetic resonance imaging procedure for preoperative mapping of cortical area associated with tactile, motor, language, and visual functions. Neurosurg. 2000;47(3):711-721.
- Kekhia H, Rigolo L, Norton I, Golby AJ. Special surgical considerations for functional brain mapping. Neurosurg Clini N Am. 2011;22(2):111-132.
- Kokkonen SM, Nikkinen J, Remes J, et al. Preoperative localization of the sensorimotor area using independent component analysis of resting-state fMRI. Magn Reson Imaging. 2009; 27(6):733-740.
- Lu JF, Zhang H, Wu JS, et al. “Awake” intraoperative functional MRI (ai-fMRI) for mapping the eloquent cortex: Is it posible in awake craniotomy? Neuroimage Clin. 2012;2:132-142.
- Kelly PJ, Daumas-Duport C, Scheithauer BW, et al. Stereotactic histologic correlations of computed tomography and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clinic Proc. 1987;62(6):450-459.
- Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30(9):907-911.
- Wallner KE, Gallcich JH, Krol G, et al. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405-1409.
- Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004; 22(9):1583-1588.
- Dincoglan F, Beyzadeoglu M, Sager O, et al. Management of patients with recurrent glioblastoma using hypofractionated stereotactic radiotherapy. Tumori. 2015;101(2):179-184.
- Niranjan A, Kano H, Lyer A, et al. Role of adjuvant or salvage radiosurgery in the management of unresected residual or progressive glioblastoma multiforme in the pre-bevacizumab era. J Neurosurg. 2015;122:757-765.
- Tsao MN, Mehta MP, Whelan TJ, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for malignant glioma. Int J Radiat Oncol Biol Phys. 2005;63(1):47-55.
- Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853-860.
- Prados MD, Wara WM, Sneed PK, et al. Phase III trial of accelerated hyperfractionation with or without difluromethylornithine (DMFO) versus standard fractionated radiotherapy with or without DMFO for newly diagnosed patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;49:71-77.
- Wilson CB, Gutin P, Boldrey EB, et al. Single agent chemotherapy of brain tumors. A five-year review. Arch Neurol. 1976;33(11):739-744.
- Boiardi A. PCV chemotherapy for recurrent glioblastoma multiforme. Neurology. 2001;56(12):1782.
- Kapelle AC, Postma TJ, Taphoorn MJ, et al. PCV chemotherapy fir recurrent glioblastoma multiforme. Neurology. 2001;56(1):118-120.
- Schmidt F, Fischer J, Herrlinger U, et al. PCV chemotherapy for recurrent glioblastoma. Neurology. 2006;66(4):587-589.
- Korones DN, Smith A, Foreman N, et al. Temozolemide and oral VP-16 for children and young adults with recurrent or treatment-induced malignant gliomas. Pediatr Blood Cancer. 2006;47(1):37-41.
- Francesconi AB, Dupre S, Matos M, et al. Carboplatin and etoposide combined with bevacizumab for the treatment of recurrent glioblastoma multiforme. J Clin Neurosci. 2010;17(8):970-974.
- Mrugala MM, Crew LK, Fink JR, et al. Carboplatin and bevacizumab for recurrent malignant glioma. Oncol Lett. 2012;4(5):1082-1086.
- Brandes AA, Ermani M, Turazzi S, et al. Procarbazine and high-dose tamoxifen as a second-line regimen in recurrent high-grade gliomas: a phase II study. J Clin Oncol. 1999;17(2):645-650.
- Brandes AA, Tosoni A, Amistá P, et al. How effective is BCNU in recurrent glioblastoma in the modern era? A phase II trial. Neurology. 2004;63(7):1281-1284.
- Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–1018.
- Pletsas D, Garelnabi EA, Li L, et al. Synthesis and quantitative structure-activity relationship of imidazotetrazine prodrugs with activity independent of O6-methylguanine-DNA-methyltransferase, DNA mismatch repair, and p53. J Med Chem. 2013;56(17):7120-7132.
- Brock CS, Newlands ES, Wedge SR, et al. Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res. 1998;58:4363-4367.
- Tolcher AW, Gerson SL, Denis L, et al. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88(7):1004-1011.
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
- Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807.
- Reitman ZJ, Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst. 2010;102(13):932.
- Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11(4):341.
- Reuss D, Sahm F, Schrimpf D, et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015;129(1):133-146.
- Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150.
- Jiao Y, Killela PJ, Reitman ZJ, et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3(7):709-722.
- Appin CL, Brat DJ. Molecular genetics of gliomas. Cancer J. 2014;20(1):66-72.
- Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64(9):3288-3295.
- Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100ª versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192-2002.
- Wong ET, Lok E, Swanson KD, et al. Response assessment of NovoTTF- 100A versus best physician’s choice chemotherapy in recurrent glioblastoma. Cancer Med. 2014;3:592-602.
- Wick A, Felsberg J, Steinbach JP, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25(22):3357-3361.
- Brandes AA, Tosoni A, Cavallo G, et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from gruppo italiano cooperativo di neuro-oncologia (GICNO). Br J Cancer. 2006;95(9):1155-1160.
- Balmaceda C, Peereboom D, Pannullo S, et al. Multi-institutional phase II study of temozolomide administered twice daily in the treatment of recurrent highgrade gliomas. Cancer. 2008;112(5):
- 1139-1146.
- Khan RB, Raizer JJ, Malkin MG, et al. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro Oncol. 2002;4(1):39-43.
- Perry JR, Rizek P, Cashman R, et al. Temozolomide rechallenge in recurrent malignant glioma by using a continuous temozolomide schedule: the “rescue” approach. Cancer. 2008;113(8):2152-2157.
- Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28(12):2051-2047.
- El Amrani M, Heinzlef O, Debroucker T, et al. Brain infarction following 5-fluoro-uracil and cisplatin therapy. Neurology. 1998;51:899-901.
- Weissman DE, Dufer D, Vogel V, et al. Corticosteroid toxicity in neurooncology patients. J Neurooncol. 1987;5(2):125-128.
- Dhermain FG, Hau P, Lanfermann H, et al. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9:906-920.
- Hu LS, Eschbacher JM, Heiserman JE, et al. Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro Oncol. 2012;14(7):919-930.
- Bobek-Billewicz B, Stasik-Pres G, Majchrzak H, Zarudzki L. Differentiation between brain tumor recurrence and radiation injury using perfusion, diffusion-weighted imaging and MR spectroscopy. Folia Neuropathol. 2010;48(2):81-92.
- Di Costanzo A, Trojsi F, Giannatempo GM, et al. Spectroscopic, diffusion and perfusion magnetic resonance imaging at 3.0 Tesla in the delineation of glioblastomas: preliminary results. J Exp Clin Cancer Res. 2006;25(3):383-390.
- Boxerman JL, Ellingson BM, Jeyapalan S, et al. Longitudinal DSCMRI for distinguishing tumor recurrence from pseudoprogression in patients with a high-grade glioma. Am J Clin Oncol. 2014 Nov 26.
- Kim DY, Kim HS, Goh MJ, et al. Utility of intravoxel incoherent motion MR imaging for distinguishing recurrent metastatic tumor from treatment effect following gamma knife radiosurgery: initial experience. Am J Neuroradiol. 2014;35(11):2082-2090.
- Verma N, Cowperthwaite MC, Burnett MG, Markey MK. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol. 2013; 15(5):515-534.
- Zeng Q-S, Li CF, Liu H, et al. Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys. 2007;68(1):151-158.
- Smith EA, Carlos RC, Junck LR, et al. Developing a clinical decision model: MR spectroscopy to differentiate between recurrent tumor and radiation change in patients with new contrast-enhancing lesions. Am J Roentgenol. 2009;192(2):W45-W52.
- Schlemmer HP, Bachert P, Herfarth KK. Proton MR spectroscopic evaluation of suspicious brain lesions after stereotactic radiotherapy. AJNR Am J Neuroradiol. 2001;22(7):1316-1324.
- Plotkin M, Eisenacher J, Bruhn H, et al. 123I-IMT SPECT and 1H MR-spectroscopy at 3.0 T in the differential diagnosis of recurrent or residual gliomas: a comparative study. J Neurooncol. 2004;70(1):49-58.
- Elias AE, Carlos RC, Smith EA, et al. MR spectroscopy using normalized and non-normalized metabolite ratios for differentiating recurrent brain tumor from radiation injury. Acad Radiol.
2011;18(9): 1101-1108. - Guo J, Yao C, Chen H, et al. The relationship between Cho/NAA and glioma metabolism: implementation for margin delineation of cerebral gliomas. Acta Neurochir (Wien). 2012;154(8): 1361-1370.
- Nihashi T, Dahabreh IJ, Terasawa T. PET in the clinical management of glioma: evidence map. Am J Roentgenol. 2013;200:W654-W660.
- Gulyas B, Halldin C. New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q J Nucl Med Mol Imaging. 2012;56:173-190.
- Juhasz C, Dwivedi S, Kamson DO, et al. Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors. Mol Imaging. 2014;13.
- Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49:694-699.
- Nariai T, Tanaka Y, Wakimoto H, et al. Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg. 2005;103:498-507.
- Rachinger W, Goetz C, Popperl G et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57:505-511.
- Weber WA, Wester HJ, Grosu AL, et al. O-(2-[18F]fluoroethyl)-L-tyrosine and L-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med. 2000;27:542-549.
- Grosu AL, Astner ST, Riedel E, et al. An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys. 2011;81:1049-1058.
- Galldiks N, Dunkl V, Stoffels G, et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2- [18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015; 42:685-695.
- Guyotat J, Signorelli F, Frappaz D, et al. Is reoperation for recurrence of glioblastoma justified? Oncol Rep. 2000;7:899-904.
- Bloch O, Han SJ, Cha, S, et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117:
- 1032-1038.
- McNamara MG, Lwin Z, Jiang H, et al. Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J Neurooncol. 2014;117:147-152.
- Woernle CM, Peus D, Hofer S, et al. Efficacy of surgery and further treatment of progressive glioblastoma. World Neurosurg. 2015;84(2):301-307.
- Franceschi E, Bartolotti M, Tosoni A, et al. The effect of re-operation on survival in patients with recurrent glioblastoma. Anticancer Res. 2015;35: 1743-1748.
- Park JK, Hodges T, Arko L, et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol. 2010;28:3838-3843.
- Park CK, Kim JH, Nam DH, et al. A practical scoring system to determine whether to proceed with surgical resection in recurrent glioblastoma. Neuro Oncol. 2013;15:1096-1101.
- Ringel F, Pape H, Sabel M, et al. Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. (Aug 4. pii: nov145. [Epub ahead of print]).
- Ryu S, Buatti JM, Morris A, et al. The role of radiotherapy in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118:489-499.
- Combs SE, Debus J, Schulz-Ertner D. Radiotherapeutic alternatives for previously irradiated recurrent gliomas. BMC Cancer. 2007;7:167.
- Barnett GH, Linskey ME, Adler JR, et al. Stereotactic radiosurgery—an organized neurosurgery-sanctioned definition. J Neurosurg. 2007;106:1-5.
- Larson EW, Peterson HE, Lamoreaux WT, et al. Clinical outcomes following salvage Gamma Knife radiosurgery for recurrent glioblastoma. World J Clin Oncol. 2014;5:142-48.
- Skeie BS, Enger PO, Brogger J, et al. Gamma Knife surgery versus reoperation for recurrent glioblastoma multiforme. World Neurosurg. 2012;78:658-669.
- Park KJ, Kano H, Iyer A, et al. Salvage Gamma Knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: a case-control study. J Neurooncol. 2012; 107:323-333.
- Koga T, Maruyama K, Tanaka M, et al. Extended field stereotactic radiosurgery for recurrent glioblastoma. Cancer. 2012;118:4193-4200.
- Elliott RE, Parker EC, Rush SC, et al. Efficacy of gamma knife radiosurgery for small-volume recurrent malignant gliomas after initial radical resection. World Neurosurg. 2011;76:128-140.
- Pouratian N, Crowley RW, Sherman JH. Gamma Knife radiosurgery after radiation therapy as an adjunctive treatment for glioblastoma. J Neurooncol. 2009;94:409-418.
- Kida Y, Yoshimoto M, Hasegawa T. Radiosurgery for intracranial gliomas. In: Yamamoto M, ed. Japanese Experience with Gamma Knife Radiosurgery. Basel: Karger; 2009:122-128.
- Kong DS, Lee JI, Park K, et al. Efficacy of stereotactic radiosurgery as a salvage treatment for recurrent malignant gliomas. Cancer. 2008;112: 2046-2051.
- Kohshi K, Yamamoto H, Nakahara A, et al. Fractionated stereotactic radiotherapy using gamma unit after hyperbaric oxygenation on recurrent high-grade gliomas. J Neurooncol. 2007;82:297-303.
- Hsieh PC, Chandler JP, Bhangoo S, et al. Adjuvant gamma knife stereotactic radiosurgery at the time of tumor progression potentially improves survival for patients with glioblastoma multiforme. Neurosurgery. 2005;57:684-692.
- Field KM, Simes J, Nowak AK, et al. Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015;17:
- 1504-1013.
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009; 27:
- 4733-4740.
- Li J, Huang S, Armstron EA, et al. Angiogenesis and radiation response modulation after vascular endotelial growth factor receptor-2 (VEGFR2) blockade. Int J Radiat Oncol Biol Phys. 2005;62(5):1477-1485.
- Kleibeuker EA, Griffioen AW Verheul HM, et al. Combining angiogenesis inhibition and radiotherapy: a doubleedged sword. Drug Resist Updat. 2012;15(3):173-182.
- Wick W, Brandes A, Gorlia T, et al. Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro Oncol. 2015;17:suppl 5;LB05.
- Davis L. Spongioblastoma multiforme of the brain. Ann Surg. 1928;87:8-14.
- Smith DR, Hardman JM, Earle KM. Metastasizing neuroectodermal tumors of the central nervous system. J Neurosurg. 1969;31:50-58.
- Pasquier B, Pasquier D, N’Golet A, et al. Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer. 1980;45:112-125.
- Datta CK, Weinstein JD, Bland JE, et al. A case of cervical lymph node metastasis resulting from glioblastoma multiforme. W V Med J. 1998;94: 276278.
- Fecteau AH, Penn I, Hanto DW. Peritoneal metastasis of intracranial glioblastoma via a ventriculoperitoneal shunt preventing organ retrieval: case report and review of the literature. Clin Transplant. 1998;12:348350.
- Piccirilli M, Brunetto GM, Rocchi G, et al. Extra central nervous system metastases from cerebral glioblastoma multiforme in elderly patients. Clinicopathological remarks on our series of seven cases and critical review of the literature. Tumori. 2008;94:4051.
- Templeton A, Hofer S, Töpfer M, et al. Extraneural spread of glioblastoma report of two cases. Onkologie. 2008;31:192194.
- Widjaja A, Mix H, Gölkel C, et al. Uncommon metastasis of a glioblastoma multiforme in liver and spleen. Digestion. 2000;61:219222.
- Yasuhara T, Tamiya T, Meguro T, et al. Glioblastoma with metastasis to spleen case report. Neurol Med Chir (Tokyo). 2003;43:452456.
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-466.
- Curran WJ, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704-710.
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352: 997-1003.
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987996.
- Stupp R, Wong E, Scott C et al. Interim analysis of the EF-14 trial: a prospective, multi-center trial of NovoTTF-100A together with temozolamide compared to temozolamide alone in patients with newly diagnosed GBM. Neuro Oncol. 2014;16:V167.
- Adkison J, Tomé W, Seo S, et al. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(3):835-841.
- Stupp R, Hegi M, Mason W, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-466.
- National Cancer Institute. http://www.cancer.gov/about-cancer/treatment/clinical-trials/search/results?protocolsearchid=6205688. Accessed February 23, 2016.
Citation
Sanchez LM. Glioblastoma: Multidisciplinary treatment approaches. Appl Radiat Oncol. 2016;(1):17-25.
March 7, 2016
