Indications, barriers and paths to advancement in palliative radiation oncology
Images
SA-CME credits are available for this article here.
Radiation therapy (RT) has been used successfully for cancer symptom palliation for more than a century in a time-efficient and cost-effective manner for palliative care delivery, even when clinicians had an incomplete understanding of its mechanism of action. Shortly after the 1895 discovery of the x-ray by Wilhelm Roentgen, radiation’s paramount use became treating cancer-related symptoms.1 Palliative radiation dose depends on overall patient condition including prognosis, performance status, prior treatment, comorbid conditions, risk of acute toxicity, and concurrent systemic therapy, and is delivered taking into account patient wishes.2 Palliative treatment courses of 8 to 30 Gy × 1 to 10 fractions are commonly used for a wide range of scenarios, although other fractionation schemes also exist. Careful selection of dose, time and fractionation is important in palliative patients with limited life expectancies. High-dose-per-fraction or hypofractionated treatments may correlate with a higher late toxicity risk; however, per linear quadratic modeling, a single 8-Gy treatment has a lower risk of late effects than 30 to 40 Gy × 10 to 20 fractions. Similarly, higher acute toxicity is associated with a course of 30 Gy × 10 fractions compared with a single 8 Gy fraction.3
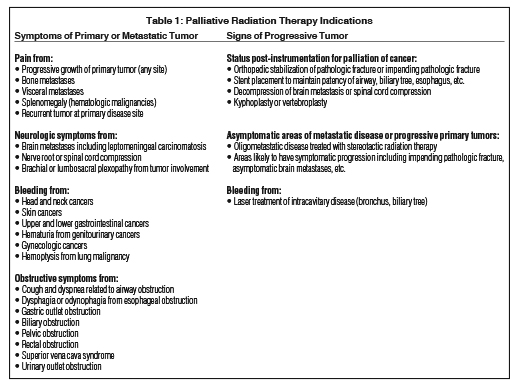
The benefits of palliative radiation are not limited by tumor histology or anatomic site of treatment. Tumor symptoms and signs may be relieved by RT to the central nervous system, respiratory system, gastro-intestinal tract, genitourinary system, skeleton, and skin, among other areas. Although cells of malignant melanoma and renal cell carcinoma are known to repair radiation-induced damage more efficiently than other tumors, they still respond to palliative RT.1 Table 1 summarizes common indications for palliative radiation treatment.
Estimating prognosis has remained difficult for clinicians caring for patients receiving palliative RT compared with colleagues in other oncology disciplines.4 However, considerable effort has been made over the past 10 years to develop models to predict patient life expectancy. Chow and colleagues developed and validated a predictive model that determines prognosis using 3 risk factors among patients referred for palliative RT.5 They collected potential clinical prognostic factors for 395 patients, including symptoms from the Edmonton Symptom Assessment Scale, and showed that nonbreast primary, metastases to nonbony sites, and Karnofsky performance status < 60 divided patients into 3 groups (0 to 1, 2, or 3 risk factors) with remarkably different survival times. The data were further validated among an additional 445 patients from the same institution and 468 patients at a separate institution. Median survivals are 31 weeks, 13 weeks and 6 weeks for patients with 0 to 1 risk factors, 2 risk factors and 3 risk factors, respectively.6 Other prognostic models have also shown utility among patients receiving palliative RT, including the TEACHH model7 and models among patients receiving palliative RT for specific clinical scenarios, such as brain metastases8 and cord compression.9,10
Herein we discuss the most common palliative radiation oncology scenarios encountered by radiation oncologists in their routine practice: bone metastases; brain metastases; malignant spinal cord and cauda compression; and tumor-related bleeding, fungation, obstruction and visceral metastases. Next, barriers to the advancement of palliative radiation oncology, including hurdles in clinical care and research opportunities, will be considered together with strategies to overcome these barriers to benefit advanced cancer patients and their families.
Bone Metastases
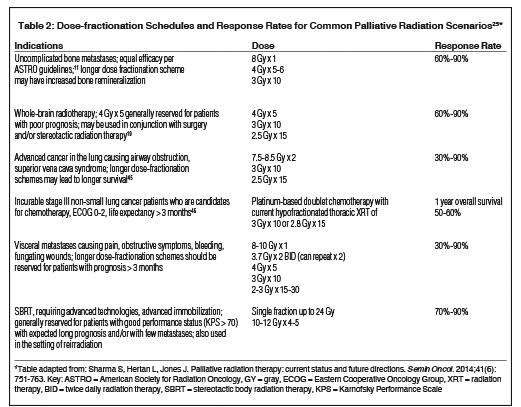
External-beam RT offers the most efficient and well-tolerated therapy for painful bony metastases when combined with suitable measures such as surgical fixation, bone-strengthening agents, radiopharmaceuticals and pain medication regimens11 with complete pain relief of 30% to 50% and partial pain relief of 60% to 80% at 3 to 4 weeks after starting palliative radiation treatment.12 Different treatment schedules for managing uncomplicated bone metastases (ie, no reirradiation, pathological fracture or cord/cauda compression) including 30 Gy × 10 fractions; 24 Gy × 6 fractions; 20 Gy × 5 fractions; and a single, 8 Gy fraction have shown equivalent pain relief in several prospective randomized trials.11 On average, re-treatment rates are 8% among patients receiving multifraction regimens and 20% among patients receiving a single fraction, with single-fraction treatment not showing any detrimental effect even when assessed for late spinal cord tolerance.13 Also, pain control was not inferior after a single 8 Gy fraction compared with protracted courses in a group of patients who survived beyond a year.14 Clinical judgment and shared decision-making with patients are recommended to determine which fractionation regimen is appropriate. A single 8 Gy fraction is a reasonable option for patients with limited life expectancy or any patients with uncomplicated painful bone metastasis. It is not certain whether hypofractionated regimens lead to better local control or prevention of fracture compared with a single 8 Gy fraction.
Excellent palliation for painful bone metastases along with safe and effective re-treatment have been confirmed by updated analyses.15 Expert and thorough judgment and discretion by radiation oncologists are crucial when deciding on fractionation and advanced techniques such as stereotactic body radiation therapy (SBRT) along with specific consideration for life expectancy; comorbid conditions; tumor biology; anatomy; previous radiation at or near the current site of treatment; tumor and normal tissue response to local and systemic therapies; and other factors relevant to the patient, tumor or treatment characteristics.15 The rapid access palliative RT programs throughout Canada have helped improve patient access to RT and in-depth study of bone metastases management.16,17 Such integrated services are becoming common, enhancing patient, family and team satisfaction and helping with prognostication, collaboration and combined decision-making.18
Brain Metastases
Brain metastases are common with multiple tumor types and are a significant cause of cancer morbidity and mortality. Treatment options are based on global patient factors, such as prognosis, and metastatic site-specific factors, such as site-related symptoms and number/burden of metastatic disease.19 For example, the use of the diagnosis-specific graded prognostic index (DS-GPA) to predict life expectancy can help tailor management of brain metastases based on performance status, age, number of brain metastases, extra-cranial metastases and cancer type.8 Thoughtful palliative care is important, as survival ranges from 2.8 to 25.3 months depending on prognostic factors. Without clear evidence of uniform preference for using local modality combinations (surgery and radiosurgery) vs whole-brain radiation therapy, it is important to consider the ideal combination for a given patient. Of note, the addition of whole-brain radiation to surgery or radiosurgery does not confer a survival advantage and can diminish quality of life and cognitive function.20 When deciding between 30 Gy × 10 fractions and 20 Gy × 5 fractions, the shorter course seems more logical in patients with short life expectancy for optimal convenience, given that no differences in overall survival or symptom control have been shown between the regimens. In some patients, particularly those with poorer prognosis, supportive care only, with dexamethasone and pain medication, is reasonable.3
In the past 1 to 2 decades, stereotactic radiosurgery (SRS) has transformed brain metastases management. Randomized controlled trials have demonstrated high local control benefits after SRS for brain oligometastatic disease, and a prospective study has shown that this may be considered for up to 10 brain metastases. Its minimally invasive nature makes it a reasonable alternative to surgical resection. Furthermore, novel targeted therapies and immunotherapies with favorable side-effect profiles allow for concurrent systemic therapy delivery with radiosurgery. Possible synergistic effects have been demonstrated, thus expediting treatment of intracranial and extracranial disease.21
Malignant Spinal Cord and Cauda Equina Compression
Malignant spinal cord and cauda equina compression is an oncologic emergency typically resulting from extraosseous extension of tumor from bones of the spine into neural structures, although the clinical scenario also can manifest due to epidural, intradural or even intramedullary metastatic disease. Pain usually predates neurological deficits by days to months, and resultant dysfunction can include motor weakness, sensory deficits and loss of bowel and bladder function. Neurological functional losses require prompt recognition and timely intervention to prevent long-term functional deficits.12
Starting corticosteroids to diminish edema is the first step in managing spinal cord/cauda compression. The next step is deciding between surgical decompression followed by RT or radiation alone. In a randomized trial, Patchell et al22 demonstrated that surgical decompression followed by RT (3 Gy × 10) leads to improved ambulation when compared with radiation (3 Gy × 10) alone for patients with a single site of metastatic epidural spinal cord compression (SCC) from different tumors and a good performance status. Patients undergoing surgical decompression were more likely to maintain ambulatory status, although that benefit decreased with age.23 Patients treated with RT alone generally respond to multifraction regimens such as 3 Gy × 10 fractions, although recent literature suggests that patients with short life expectancy do well with a single 8 Gy dose.3 Rades and colleagues prospectively followed a large cohort of patients treated with different dose-fractionation schemes (8 Gy × 1 or 4 Gy × 5 for short course or 3 Gy × 10, 2.5 Gy × 15 or 2 Gy × 20 for long course). The authors found that longer dose-fractionation schemes led to higher rates of local tumor control. This suggests that a higher biological equivalent dose is more likely to control spine tumors causing cord compression than a lower biological equivalent dose for patients with longer life expectancy.10 However, no differences in motor function change or overall survival between the groups were seen, suggesting that short-course radiation may be appropriate for patients with a life expectancy of < 3 months. In the same population, Rades et al also developed and validated a score to predict survival after development of spinal cord compression. The score is based on histology, presence of other bone metastases, presence of visceral metastases, time from initial diagnosis of cancer to development of SCC, ambulatory status at the time of SCC, and time to develop motor deficits from the onset of SCC. The score predicts 6-month survival percentages of 16% for poor prognosis, 48% for intermediate prognosis and 81% for better prognosis patients, demonstrating the possibility of tailoring RT to anticipated survival.9 Feasibility of spine radiosurgery for the treatment of SCC has been demonstrated by Ryu and colleagues;24 however, additional studies are needed to determine SRS safety in this setting given the high RT dose and proximity to the spinal cord.
Tumor-related Bleeding, Fungation, Obstructive Symptoms and Visceral Metastases
Fundamental principles of RT apply for primary tumors or metastases causing symptoms in areas beyond bone and the central nervous system (CNS), and causing pain, bleeding, open wounds, or other local symptoms specific to the affected region (eg, dysphagia in the head and neck and esophagus, cough and dyspnea in the lung, etc.). Optimal dose-fractionation schemes have not been established and usually depend on the specific clinical scenario, patient’s performance status and life expectancy. Short RT courses (including single-fraction) are more appropriate for patients with poor performance status and poor prognosis. For patients with intermediate prognosis, schedules such as 30 Gy × 10 fractions or more dose-intense hypofractionated regimens, such as the “quad shot RT” (4 fractions delivered twice a day over 2 days and repeated weekly up to 2 additional times depending on performance status and response), may be suitable.25 In patients with good performance status with no significant burden of metastatic disease and projected long survival, protracted courses of RT (eg, 40 to 60 Gy × 15 to 30 fractions) may be more appropriate. Furthermore, SBRT is increasingly being used in patients with oligometastatic disease for local control if the lesions can be treated keeping to normal tissue constraints.25
Table 2 summarizes dose fractionation schedules for common palliative RT cases along with estimated response rates.
Barriers and Opportunities in Advancing Palliative Radiation Oncology Practice
Several factors limit advancement in palliative RT clinical care and research. There is hesitancy in adopting evidence-based practice despite several large palliative trials by the Radiation Therapy Oncology Group (RTOG).26,27 Generally, palliative care outcome measures are hard to define and difficult to measure. Patient-reported, validated measures are usually the most useful outcome variables, although many commonly used instruments have not been fully validated in trials. Furthermore, many palliative radiation trials suffer from missing data points as patients are not always able to fulfill follow-up appointments due to declining function or mortality.28
In addition, few resources are being spent on palliative radiation research compared to the number of palliative oncology patients in the United States each year as well as their symptom severity. This is reflected in part by the proportion of abstracts related to palliative care and symptom control submitted to the American Society for Radiation Oncology (ASTRO) from 1993 to 2000, which accounted for about 1.3% of overall submissions.29 A more recent update on trends in the number of original scientific reports directly addressing palliative care outcomes in the Red and Green journals – two of the most prestigious and influential radiation oncology journals – showed minimal change in original research publications since the early 2000s.30
Resident Education
Approximately 30% to 40% of RT courses delivered are palliative in intent, and radiation oncologists are involved throughout the trajectory of advanced patient care from diagnosis to end of life in providing supportive and palliative care (SPC). As discussed, palliative RT is an important tool for maximizing patient quality of life in the face of incurable cancers. Hence, radiation oncologists are key members of an interdisciplinary oncology palliative care team.32 However, despite the extent of palliative care provision within radiation oncology, radiation oncologists frequently report not having adequate competencies in palliative care.33
Several efforts have been made to further define and assess educational needs within SPC competencies in radiation oncology. As part of the overall competency assessment of radiation oncology resident trainees, the Accreditation Council of Graduate Medical Education (ACGME) and the American Board of Radiology (ABR) published radiation oncology competency assessment milestones that included palliative oncology care as 1 of 22 competency areas. The SPC milestone includes accurate pain and nonpain symptom assessment, independent management of toxicities and symptoms associated with RT, and developing appropriate and effective palliative care management strategies.34,35 To define current SPC educational structures within residency programs, a survey-based study assessed program directors’ perspectives of palliative care education in radiation oncology residency. This study revealed that although most of them considered SPC (93%) and palliative RT (99%) as important competencies for radiation oncology residents and fellows, only 67% of residency programs had formal educational activities in palliative and supportive care principles and practice. A formal curriculum on palliative RT applications was reported in 85% of programs and mostly focused on education regarding palliative RT to the brain, bone and spine, but less commonly for visceral or skin metastasis. The majority of programs had formal didactics of 1 or more hours on pain management (67%), neuropathic pain (65%) and nausea and vomiting management (63%), whereas initial management of fatigue (35%), spirituality assessment (33%) and advance care directives discussion (30%) were less frequently addressed.36
A national survey of radiation oncology residents by Krishnan and colleagues addressed residents’ perspectives of their SPC education sufficiency across 8 generalist palliative care competency domains derived from national guidelines.37 These are: symptom management (pain, nonpain), communication about goals of care, advance care planning, psychosocial issues, cultural considerations, spiritual needs, care coordination, and ethical/legal issues. The survey assessed, within these domains, residents’ perceptions of: 1) the adequacy of their education, 2) competency in each domain, and 3) overall importance of palliative care competencies within radiation oncology. On average across the 8 domains, 79% of residents rated their training as “not at all,” “minimally,” or “somewhat” adequate. The SPC domains in which residents rated themselves as “not at all,” “minimally,” or “somewhat” confident were symptom management (36% pain, 44% nonpain), communication about goals of care (31%), advance care planning (48%), psychosocial issues (55%), cultural issues (22%), spiritual issues (44%), care coordination (50%) and legal/ethical issues (50%). Palliative care was perceived as an important competency for radiation oncologists by 96% of residents and greater SPC education was desired by 81%. The importance of improving generalist palliative care education in oncology has been emphasized in expert consensus recommendations.32,38,39 Several randomized trials have demonstrated improved patient outcomes through the integration of palliative care for oncology patients.40,41 For example, in a randomized study among 151 advanced lung cancer patients, Temel et al found that despite receiving less aggressive medical care at the end of life, early palliative care was associated with improved quality of life and survival with reduced depression.42 Also, early palliative care is associated with reduced costs of end-of-life medical care.43
Incorporating specialty palliative care in oncology care is now recommended for all advanced cancer patients by the American Society of Clinical Oncology (ASCO) based on the aforementioned evidence.32 In these guidelines, oncologists are urged to be trained in, and provide, generalist palliative care to their patients. This is necessary due to oncologists’ regular role in meeting generalist palliative care needs, such as managing ongoing symptoms, discussing goals of care as part of treatment decision-making, and identifying nonadvanced cancer patients in need of palliative care specialty referrals. Additionally, at present there are insufficient specialty palliative care resources to meet the care needs as presented in the ASCO guidelines, particularly as many patients with advanced cancer are living longer due to advances in systemic therapies, such as immunotherapies. Such factors underscore the need for robust generalist palliative care education for all oncology-related disciplines, including radiation oncologists, who frequently are involved in care for patients with advanced cancers.44 Given the frequency of patients presenting to radiation oncology with complex palliative care issues such as significant pain syndromes and difficult end-of-life medical decision-making, there is a clear need to improve education across generalist palliative oncology care domains in radiation oncology training. Likewise, increasing the quantity and quality of radiation oncology resident training in palliative care should be emphasized.37 Given that many radiation oncologists have no formal training in hospice and palliative care during training and residency, it is also critical that high-quality palliative RT topics be presented at radiation oncology clinical meetings.
While this article focuses on the RT aspect of palliative care, the scope of palliative care is much wider. Important aspects of palliative care practice that may be integrated into residency training include decisions on when to offer treatment, limits of palliative RT, goals of care discussion, open and empathic communication with patients and family about prognosis, and facilitation of care to hospice or a nursing facility. Working with other specialists including medical oncology and palliative care on these diverse but complicated issues offers the maximal opportunity to define optimal care for symptom management and to improve the quality of life of patients with advanced cancer and their family. A case synopsis below illustrates an approach integrating a palliative radiation plan in the overall goals of care.
Conclusion
Palliative care is an integral part of radiation oncology practice, and radiation oncologists must be facile with the best evidence-based palliative RT applications in common clinical scenarios, including bone metastases, brain metastases, malignant spinal cord and cauda equina compression, tumor-related bleeding, fungation, obstruction and visceral metastases. Further rigorous research is needed to define technical aspects of palliative RT delivery, such as in the application of advanced techniques (eg, SBRT). However, supportive and palliative care competencies must extend beyond the technical aspects of RT delivery to generalist palliative care competencies, including the basics of symptom management, communication and goals of care, advance care planning, psychosocial issues, cultural considerations, spiritual needs, and ethical/legal issues. Radiation oncologists must also interface with specialty palliative care teams, recognizing when referrals are needed, and acting as part of the interdisciplinary oncology palliative care team. Together with greater research, further education is needed both within and beyond residency training to best equip radiation oncologists to advance the care of cancer patients living with incurable disease.
CASE SYNOPSIS
Mr. H is a 97-year-old man with a history of multiple comorbid conditions and diffuse large B cell lymphoma (DLBCL), status post R-CHOP x 3 and involved-field radiation therapy (IFRT) (R axilla) in 2015. He transferred his care to our hospital after being admitted at an outside hospital with severe back pain without adequate control despite narcotics. Imaging evaluation showed a paraspinal mass invading the T8-10 without a compression fracture. Biopsy of this mass revealed DLBCL but was complicated by acute lower extremity weakness. Spine magnetic resonance imaging (MRI) revealed a hematoma at the epidural space at the biopsy site, without cord compression. Computed tomography (CT) of the chest, abdomen and pelvis demonstrated extensive mediastinal and retroperitoneal adenopathy. Radiation oncology was consulted for urgent palliative radiation to the lower thoracic spine. Upon assessment, the elderly patient appeared to be in very poor health and had not been ambulated due to progressive generalized weakness. His performance status was significantly declining in the last 6 months.
RECOMMENDATION:
Medical intervention with narcotic and nonsteroidal anti-inflammatory medications.
Consider a single fraction of 8 Gy to the painful paraspinal mass.
The patient refused radiation treatment, but the son, the health care proxy, wanted to pursue every possible intervention, including RT.
RECOMMENDATION:
Review cancer treatment options. Multidisciplinary discussion and communication with patient and family about prognosis and goals of care.
Communication with family members to understand medical and psychosocial concerns.
Multiple daily discussions with the inpatient team and son took place to discuss the goals of care and expected objectives to palliate symptoms. Consensus was reached with the patient proceeding to inpatient hospice care. The patient received a single 8 Gy fraction of palliative RT to the midthoracic spine.
CASE QUESTIONS
What is the frequency of complex palliative care issues, such as psychosocial, ethical and goals of care issues, relevant to our care of patients?
Parker et al31 conducted a survey-based study of radiation oncology clinicians seeing 163 consecutive patients for urgent palliative RT. Most (82%) consults had 2 or more palliative care domains ranked as highly relevant to care that included physical symptoms (91%), care coordination (70%), goals of care (59%), and psychosocial issues (52%).
References
- Lutz S, Korytko T, Nguyen J, et al. Palliative radiation therapy: when is it worth it and when is it not? Cancer J. 2010;16(5):473-482.
- Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6-9.
- Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer. J Clin Oncol. 2014;32(26):2913-19.
- Gripp S, Mjartan S, Boelke E, Willers R. Palliative radiation therapy tailored to life expectancy in end-stage cancer patients: reality or myth? Cancer. 2010;116(13):3251-3256.
- Chow E, Abdolell M, Panzarella T, et al. Predictive model for survival in patients with advanced cancer. J Clin Oncol. 2008;26(36):5863-5869.
- Chow E, Abdolell M, Panzarella T, et al. Validation of a predictive model for survival in metastatic cancer patients attending an outpatient palliative radiation therapy clinic. Int J Radiat Oncol Biol Phys. 2009;73(1):280-287.
- Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectancy in patients with metastatic cancer receiving palliative radiation therapy: the TEACHH model. Cancer. 2014;120(1):134-141.
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419-425.
- Rades D, Douglas S, Veninga T, et al. Validation and simplification of a score predicting survival in patients irradiated for metastatic spinal cord compression. Cancer. 2010;116(15):3670-3673.
- Rades D, Lange M, Veninga T, et al. Final results of a prospective study comparing the local control of short-course and long-course radiation therapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2011;79(2):524-530.
- Lutz S, Berk L, Chang E, et al. Palliative radiation therapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79(4):965-976.
- Chow E, Harris K, Fan G, et al. Palliative radiation therapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423-1436.
- Howell DD, James JL, Hartsell WF, et al. Single-fraction radiation therapy versus multifraction radiation therapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer. 2013;119(4):888-896.
- van der Linden YM, Steenland E, van Houwelingen HC, et al. Patients with a favourable prognosis are equally palliated with single and multiple fraction radiation therapy: results on survival in the Dutch Bone Metastasis Study. Radiother Oncol. 2006;78(3):245-253.
- Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7(1):4-12.
- Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiation therapy program to reduce waiting time for palliative radiation therapy. Support Care Cancer. 2006;14(1):38-43.
- Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiation therapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
- Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
- Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
- Wolf A, Kondziolka D. Brain metastases: radiosurgery. Handb Clin Neurol. 2018;149:129-135.
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
- Chi JH, Gokaslan Z, McCormick P, et al. Selecting treatment for patients with malignant epidural spinal cord compression-does age matter? Results from a randomized clinical trial. Spine (Phila Pa 1976). 2009;34(5):431-435.
- Ryu S, Fang Yin F, Rock J, et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97(8):2013-2018.
- Sharma S, Hertan L, Jones J. Palliative radiation therapy: current status and future directions. Semin Oncol. 2014;41(6):751-763.
- Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiation therapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
- Murray KJ, Scott C, Zachariah B, et al. Importance of the mini-mental status examination in the treatment of patients with brain metastases: a report from the Radiation Therapy Oncology Group protocol 91-04. Int J Radiat Oncol Biol Phys. 2000;48(1):59-64.
- Lutz S, Lupu D, Johnstone P, et al. The influence of the newly formed hospice and palliative medicine subspecialty on radiation oncology and end-of-life care. J Am Coll Radiol. 2008;5(11):1102-1105.
- Barnes EA, Palmer JL, Bruera E. Prevalence of symptom control and palliative care abstracts presented at the Annual Meeting of the American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2002; 54(1):211-214.
- Shi DD, Digiovani JD, Skamene S, et al. Patterns of symptom control and palliative care-focused original research articles in major radiation oncology academic journals. Ann Palliat Med. 2018; In press.
- Parker GM, LeBaron VT, Krishnan M, et al. Burden of palliative care issues encountered by radiation oncologists caring for patients with advanced cancer. Pract Radiat Oncol. 2017;7(6):e517-524.
- Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
- Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: Report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7:113-119.
- Weissman DE, Block SD. ACGME requirements for end-of-life training in selected residency and fellowship programs: a status report. Acad Med. 2002;77(4):299-304.
- The radiation oncology milestone project. J Grad Med Educ. 2014;6(1 Suppl 1):307-316.
- Wei RL, Colbert LE, Jones J, et al. Palliative care and palliative radiation therapy education in radiation oncology: a survey of US radiation oncology program directors. Pract Radiat Oncol. 2017;7(4):234-240.
- Krishnan M, Racsa M, Jones J, et al. Radiation oncology resident palliative education. Pract Radiat Oncol. 2017;7(6):e439-448.
- Peppercorn JM, Smith TJ, Helft PR, et al. American society of clinical oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29(6):755-760.
- Dying in America: improving quality and honoring individual preferences near the end of life. Mil Med. 2015;180(4):365-367.
- Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med. 2004;164(1):83-91.
- Rummans TA, Clark MM, Sloan JA, et al. Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: a randomized controlled trial. J Clin Oncol. 2006; 24(4):635-642.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
- Scibetta C, Kerr K, McGuire J, Rabow MW. The costs of waiting: implications of the timing of palliative care consultation among a cohort of decedents at a comprehensive cancer center. J Palliat Med. 2016;19(1):69-75.
- Schaefer KG, Chittenden EH, Sullivan AM, et al. Raising the bar for the care of seriously ill patients: results of a national survey to define essential palliative care competencies for medical students and residents. Acad Med. 2014;89(7):1024-1031.
- Rodrigues G, Videtic GMM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: an American Society for Radiation Oncology evidence-based clinical practice guideline. Prac Radiat Oncol. 2011;1(2):60-71.
- Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiotherapy for non-small cell lung cancer: 2018 update of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018;S1879-8500(18):30069-9.
Citation
Fareed MM, Krishnan M, Balboni TA, Yu HHM. Indications, barriers and paths to advancement in palliative radiation oncology. Appl Radiat Oncol. 2018;(2):18-25.
June 19, 2018
