Paraganglioma of the skull base treated with intensity-modulated radiation therapy
Images
CASE SUMMARY
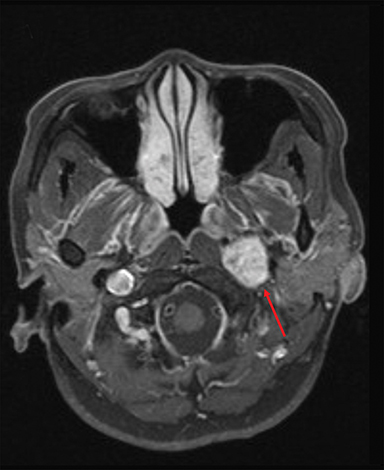
A healthy 56-year-old Vietnamese woman presented in January 2009 with a 1-year history of left-sided hearing loss and tinnitus, intermittent dizziness, and hoarseness. Audiometry evaluation revealed bilateral low-frequency hearing loss that was more severe on the left. Office nasopharyngoscopy showed a slightly hypomobile left vocal cord. An MRI of the brain with contrast revealed a 2.2 × 1.8 × 6.0-cm heterogeneously enhancing left skull base mass in the transverse (TV), anteroposterior (AP), and cranial-caudal (CC) dimensions, respectively (Figure 1A). Somatostatin receptor scintigraphy confirmed increased radiotracer uptake in the skull base lesion. The patient was clinically diagnosed with a paraganglioma of the left skull base.
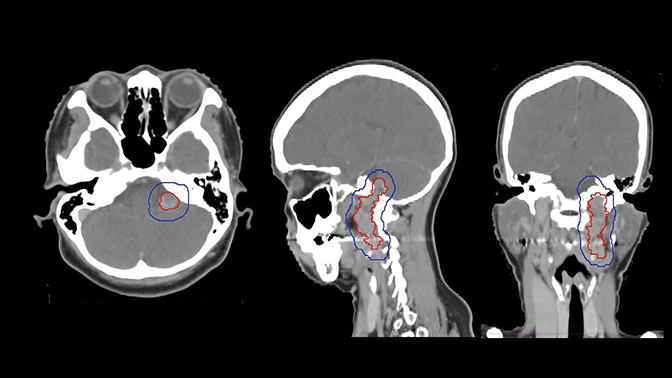
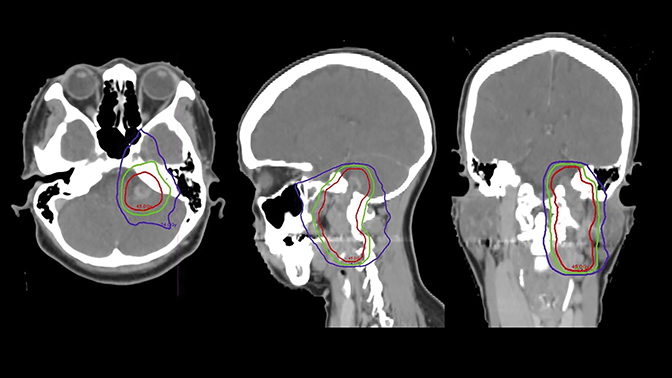
The tumor was treated definitively using intensity-modulated radiation therapy (IMRT) to a total dose of 4500 cGy in 25 fractions using 6 MV photons prescribed to the 100% isodose line between March and May of 2009, and was delivered using Novalis Tx (BrainLab, Westchester, Illinois) (Figure 2). Gross tumor volume (GTV) was defined as the index lesion as identified on CT/MRI fusion imaging. Planning target volume (PTV) was defined as an isovolumetric expansion of 7 mm on the GTV. The patient completed treatment with only grade 1 toxicities, including alopecia, fatigue and nausea.
The patient was seen for follow-up in November 2009, 6 months after completing therapy. At that time, her tinnitus improved without any radiographic changes. She did not report any grade 2 or higher toxicity.
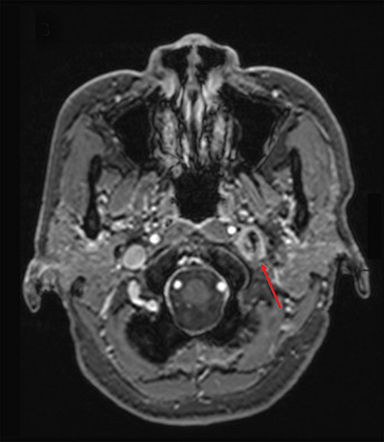
The patient was evaluated annually for follow-up until November 2013 when that changed to biennially, with her last follow-up in November 2015, 6 years after completing therapy. Throughout this period, the index lesion exhibited partial radiographic response per 2009 RECIST (Response Evaluation Criteria in Solid Tumors) criteria, with significant improvement in the cranial-caudal axis (Figure 1B). Clinically, left-sided hearing loss progressively improved over the 6 years of follow-up.
DISCUSSION
Paragangliomas are rare, benign neuroendocrine tumors arising from extra-adrenal autonomic paraganglia. Generally, parasympathetic paragangliomas are found in the neck and skull base and are nonsecretory. They are commonly found along cranial nerve IX and X, the middle ear, the jugular vein, and the carotid bodies.
The natural growth of paragangliomas has been reported to be 0.8 mm per year with a doubling time of 14 years.1 Although these tumors have an indolent course, paragangliomas can be locally invasive and cause mass effect on nearby neck structures, especially the cranial nerves, which can present as dysphagia, hoarseness, pulsatile tinnitus, and hearing loss. Larger lesions can be palpated as a rubbery, nontender mass in the lateral neck and may cause a bruit if there is mass effect on the carotid vessels. Jugulotympanic lesions can result in a bluish pulsating mass behind the tympanic membrane. A variety of other pathologies can be considered with presentation of a head and neck mass: aneurysms of the carotid artery, lymphadenopathy, head and neck malignancies, branchial cleft cysts, meningiomas, schwannomas, and thyroid nodules.
Diagnosis typically consists of biochemical and/or imaging modalities. Characteristic findings on computed tomography imaging include homogeneous mass with intense contrast enhancement. Characteristic findings on MRI include intense hypervascular appearance and classic “salt and pepper” representing hemorrhage and flow voids, respectively, on T2-weighted sequences. Somatostatin receptor scintigraphy utilizes indium-111 labeled pentetreotide, a somatostatin analog, which binds avidly in neuroendocrine tumors and can be detected with single-photon emission computed tomography imaging. Although tissue diagnosis is definitive, many of these lesions are not biopsied prior to resection either due to large size or active secretion. Thus, somatostatin scintigraphy can be used to localize and differentiate neuroendocrine tumors from other more common head and neck malignancies.
The treatment goal for a paraganglioma consists of achieving cure as defined by durable radiographic or clinical stability with avoidance of critical neurovascular structures and absence of local disease progression. Management options include observation, surgery or radiation therapy.
Observation is ideal for patients who are asymptomatic or have smaller lesions (< 2-3 cm), taking advantage of the disease’s indolent natural history. Surgical resection is used as definitive treatment for complete removal of paragangliomas. General indications for surgery include any lesions below the neck, or large skull base/neck tumors (> 3 cm), secreting or symptomatic. Surgical cure rates have been reported up to 89% to 100%, but with a significant presence of cranial nerve neuropathy.2,3 Pooled estimates have shown toxicity in CN XI/IX to be more common (40% and 38%, respectively), and less common in CN X/XII (26% and 18%, respectively).4
In the last few decades, radiation therapy has come to the forefront of managing paragangliomas with the goal of maximizing local control and minimizing damage to neurovascular structures. To date, published data on the use of radiation therapy consists of retrospective data or single institution experiences utilizing different radiation techniques. Durable radiographic or clinical stability is reported > 90% for conventional radiation therapy with similar biological effective dose, as in our case (45 Gy in 25 fractions). This is essentially equivalent to surgical local control rates with good long-term follow-up and no severe complications.3,5
Modern, highly conformal radiation techniques such as IMRT, stereotactic radiation therapy (SRT), and stereotactic radiosurgery (SRS) allow for less normal tissue toxicity and have proven to be just as efficacious, with pooled estimates showing local control rates > 90% with concomitant symptom control.4,6,7 Combs et al recently reported a single institution experience treating 39 patients with fractionated SRT, SRS, or IMRT at 18 Gy in single fraction treatment or median total dose of 57.6 Gy (at 1.8 or 2 Gy per fraction) with median follow-up of 127 months. Actuarial 10-year local control was reported at 97% with no severe long-term side effects or secondary malignancies.6
With SRS via linear accelerator, Gamma Knife (Elekta, Stockholm, Sweden), or CyberKnife (Accuray, Sunnyvale, California) modalities, there is a potential risk for damage to nearby structures secondary to large single-dose radiation; however, it has been shown that the rate of cranial nerve morbidity is vastly improved at < 12% vs. 18% to 40% after gross total resection in pooled estimates.4 Typical single-fraction dose has been quoted to be 13-20 Gy in the literature.6
Overall, radiation therapy and SRS provide equivalent local control compared to surgery, with the benefit of decreased morbidity.4,6 Selection of treatment options is often based on institutional bias and clinical preference. Nonsurgical candidates or those with high potential for neurovascular morbidity may benefit from radiation therapy, typically IMRT. SRS is usually reserved for smaller lesions (< 3 cm) given the risk of a marginal miss or for those who cannot tolerate a fractionated course. Although a randomized controlled trial would allow for an objective comparison of surgery vs. radiation therapy/SRS as primary treatment, this is unlikely given the low incidence of this condition and strong opinions regarding best management.
CONCLUSION
A 56-year-old woman presented with a 1-year history of hearing loss/tinnitus, intermittent dizziness and hoarseness. MRI of the brain revealed a 2.2 × 1.8 × 6.0-cm heterogeneous mass in the infratemporal region below the skull base within the jugular foramen. With positive uptake on somatostatin receptor scintigraphy, the presumed diagnosis was a paraganglioma of the skull base. She was treated with IMRT to 4500 cGy in 25 fractions using 6 MV photons. The patient reported only grade 1 side effects at the end of therapy. She was last seen at a 6-year follow-up when she was nearly asymptomatic, and a previous MRI at a 4-year follow-up, which showed a progressively smaller 1.3 × 2.0 × 3.0-cm mass.
Historically, surgery was the gold standard in definitive treatment for skull base/neck paragangliomas. Although cure rates were excellent, there was a significant rate of cranial nerve morbidity, especially with skull base/neck structures. Here we report a case of a skull base/neck paraganglioma that was treated with IMRT and achieved local control with 6 years of follow-up.
REFERENCES
- Jansen JC, van den Berg R, Kuiper A, et al. Estimation of growth rate in patients with head and neck paragangliomas influences the treatment proposal. Cancer. 2000;88(12):2811-2816.
- Gottfried ON, Liu JK, Couldwell WT. Comparison of radiosurgery and conventional surgery for the treatment of glomus jugulare tumors. Neurosurg focus. 2004;17(2):E4.
- Hinerman RW, Amdur RJ, Morris CG, et al. Definitive radiotherapy in the management of paragangliomas arising in the head and neck: a 35-year experience. Head Neck. 2008;30(11):1431-1438.
- Ivan ME, Sughrue ME, Clark AJ, et al. A meta-analysis of tumor control rates and treatment-related morbidity for patients with glomus jugulare tumors. J Neurosurg. 2011;114(5):1299-1305.
- Gilbo P, Morris CG, Amdur RJ, et al. Radiotherapy for benign head and neck paragangliomas: a 45-year experience. Cancer. 2014;120(23):3738-3743.
- Combs SE, Salehi-Allameh B, Habermehl D, et al. Clinical response and tumor control based on long-term follow-up and patient-reported outcomes in patients with chemodectomas of the skull base and head and neck region treated with highly conformal radiation therapy. Head Neck. 2014;36(1):22-27.
- Guss ZD, Batra S, Limb CJ, et al. Radiosurgery of glomus jugulare tumors: a meta-analysis. Internat J Radiat Oncol Biol Phys. 2011;81(4):e497-502.
